Working Group 2 - Chapter 1: Assessment of Observed Changes and Responses in Natural and Managed Systems - (AR4-WG2-1)
Original at: http://www.ipcc.ch/publications_and_data/ar4/wg2/en/ch1.html
Main AR4 Index | Working Group WG2 Index | Table of Contents | Authors | Executive Summary | Annotated Text | References | Reviewer Comments
With the exception of Chapter and Section headings, all coloured text has been inserted by AccessIPCC. The non-coloured text is the IPCC original.
A number of emails from the Climate Research Unit (CRU) of the University of East Anglia were published on the Internet in November 2009. This has provided a window into the world of climate science.
We have identified a number of key individuals involved in the emails whom we have designated as Persons of Concern [PoC]; a Journal in which a PoC has published has been designated as a Journal of Concern [JoC].
This is not to suggest that we believe such papers are necessarily flawed, but rather that, as Joseph Alcamo noted at Bali in October 2009, "as policymakers and the public begin to grasp the multi-billion dollar price tag for mitigating and adapting to climate change, we should expect a sharper questioning of the science behind climate policy".
References occur in a list at the end of each chapter. Citations are within the normal text of sections and paragraphs.
| Tag | Explanation | Where Used | References | Citations |
|---|---|---|---|---|
| PoC |
Person of Concern Key individual involved in CRU emails as defined in this spreadsheet. |
References, Citations, IPCC Roles | 10 | 24 |
| JoC |
Journal of Concern A Journal which has published articles by one or more PoCs (Person of Concern) |
References, Citations | 198 | 230 |
| MoS |
Model or Simulation Reference appears to be a model or simulation, not observation or experiment |
References, Citations | 33 | 37 |
| NPR |
Non Peer Reviewed Reference has no Journal or no Volume or no Pages or it has Editors. |
References, Citations | 114 | 132 |
| SRC |
Self Reference Concern Author of a chapter containing references to own work. |
References, Citations, IPCC Roles | 93 | 128 |
| ARC |
Paper authored or co-authored by person who is also in list of Authors of another chapter. |
References, Citations | 54 | 66 |
| 2007 |
Paper dated 2007, when IPCC policy stated cutoff was December 2005 |
References, Citations | 13 | 40 |
| Ambiguous |
The short inline citation matched with more than one reference; however, AccessIPCC will link to the first reference found. |
Citations | - | 7 |
| NotFound |
The short inline citation was not matched with any reference. Believed to be caused by typing errors. |
Citations | - | 2 |
| Clean |
The reference was probably peer reviewed. |
References, Citations | 264 | 248 |
Coordinating Lead Authors:
Cynthia Rosenzweig (USA) [SRC:2], Gino Casassa (Chile) [SRC:3],
| Concern | Occurrence |
|---|---|
| SRC 1-4 | 2 |
| Potentially Biased Authors | 2 |
Lead Authors:
David J. Karoly (USA/Australia) [SRC:3], Anton Imeson (The Netherlands), Chunzhen Liu (China), Annette Menzel (Germany) [SRC:22], Samuel Rawlins (Trinidad, Tobago), Terry L. Root (USA) [SRC:5], Bernard Seguin (France) [SRC:1], Piotr Tryjanowski (Poland) [SRC:5],
| Concern | Occurrence |
|---|---|
| SRC >= 5 | 3 |
| SRC 1-4 | 2 |
| Potentially Biased Authors | 5 |
| Impartial Authors | 4 |
Contributing Authors:
Tarekegn Abeku (Ethiopia) [SRC:1], Isabelle Côté (Canada) [SRC:3], Mark Dyurgerov (USA), Martin Edwards (UK) [SRC:10], Kristie L. Ebi (USA), Nicole Estrella (Germany) [SRC:10], Donald L. Forbes (Canada) [SRC:2], Bernard Francou (France) [SRC:6], Andrew Githeko (Kenya) [SRC:5], Vivien Gornitz (USA) [SRC:3], Wilfried Haeberli (Switzerland) [SRC:6], John Hay (New Zealand), Anne Henshaw (USA), Terrence Hughes (Australia) [SRC:1], Ana Iglesias (Spain), Georg Kaser (Austria) [SRC:4], R. Sari Kovats (UK), Joseph Lam (China) [SRC:1], Diana Liverman (UK), Dena P. MacMynowski (USA) [SRC:1], Patricia Morellato (Brazil), Jeff T. Price (USA) [SRC:1], Robert Muir-Wood (UK), Peter Neofotis (USA), Catherine O’Reilly (USA) [SRC:2], Xavier Rodo (Spain) [SRC:2], Tim Sparks (UK) [SRC:17], Thomas Spencer (UK), David Viner (UK), Marta Vicarelli (Italy), Ellen Wiegandt (Switzerland), Qigang Wu (China) [SRC:1], Ma Zhuguo (China),
| Concern | Occurrence |
|---|---|
| SRC >= 5 | 6 |
| SRC 1-4 | 12 |
| Potentially Biased Authors | 18 |
| Impartial Authors | 15 |
Review Editors:
Lucka Kajfe?-Bogataj (Slovenia), Jan Pretel (Czech Republic), Andrew Watkinson (UK) [SRC:3],
| Concern | Occurrence |
|---|---|
| SRC 1-4 | 1 |
| Potentially Biased Authors | 1 |
| Impartial Authors | 2 |
This chapter should be cited as:
Rosenzweig, C., G. Casassa, D.J. Karoly, A. Imeson, C. Liu, A. Menzel, S. Rawlins, T.L. Root, B. Seguin, P. Tryjanowski, 2007: Assessment of observed changes and responses in natural and managed systems. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, M.L. Parry, O.F. Canziani, J.P. Palutikof, P.J. van der Linden and C.E. Hanson, Eds., Cambridge University Press, Cambridge, UK, 79-131.
Executive summary
Physical and biological systems on all continents and in most oceans are already being affected by recent climate changes, particularly regional temperature increases (very high confidence) [ 1.3 ]. Climatic effects on human systems, although more difficult to discern due to adaptation and non-climatic drivers, are emerging (medium confidence) [ 1.3 ]. Global-scale assessment of observed changes shows that it is likely that anthropogenic warming over the last three decades has had a discernible influence on many physical and biological systems [ 1.4 ].
Attribution of observed regional changes in natural and managed systems to anthropogenic climate change is complicated by the effects of natural climate variability and non-climate drivers (e.g., land-use change) [ 1.2 ]. Nevertheless, there have been several joint attribution studies that have linked responses in some physical and biological systems directly to anthropogenic climate change using climate, process and statistical models [ 1.4.2 ]. Furthermore, the consistency of observed significant changes in physical and biological systems and observed significant warming across the globe very likely cannot be explained entirely by natural variability or other confounding non-climate factors [ 1.4.2 ]. On the basis of this evidence, combined with the likely substantial anthropogenic warming over the past 50 years averaged over each continent except Antarctica (as described in the Working Group I Fourth Assessment Summary for Policymakers), it is likely that there is a discernible influence of anthropogenic warming on many physical and biological systems.
Climate change is strongly affecting many aspects of systems related to snow, ice and frozen ground (including permafrost) [ 1.3.1 ]; emerging evidence shows changes in hydrological systems, water resources [ 1.3.2 ], coastal zones [ 1.3.3 ] and oceans (high confidence) [ 1.3.4 ].
Effects due to changes in snow, ice and frozen ground (including permafrost) include ground instability in permafrost regions, a shorter travel season for vehicles over frozen roads in the Arctic, enlargement and increase of glacial lakes in mountain regions and destabilisation of moraines damming these lakes, changes in Arctic and Antarctic Peninsula flora and fauna including the sea-ice biomes and predators higher in the food chain, limitations on mountain sports in lower-elevation alpine areas, and changes in indigenous livelihoods in the Arctic (high confidence). [ 1.3.1 ]
The spring peak discharge is occurring earlier in rivers affected by snow melt, and there is evidence for enhanced glacial melt. Lakes and rivers around the world are warming, with effects on thermal structure and water quality (high confidence). [ 1.3.2 ]
The effects of sea-level rise, enhanced wave heights, and intensification of storms are found in some coastal regions – including those not modified by humans, e.g., polar areas and barrier beaches – mainly through coastal erosion [ 1.3.3.1 ]. Sea-level rise is contributing to losses of coastal wetlands and mangroves, and increased damage from coastal flooding in many areas, although human modification of coasts, such as increased construction in vulnerable zones, plays an important role too (medium confidence). [ 1.3.3.2 ]
The uptake of anthropogenic carbon since 1750 has led to the ocean becoming more acidic, with an average decrease in pH of 0.1 units. However, the effects of recent ocean acidification on the marine biosphere are as yet undocumented. [ 1.3.4 ]
More evidence from a wider range of species and communities in terrestrial ecosystems and substantial new evidence in marine and freshwater systems show that recent warming is strongly affecting natural biological systems (very high confidence). [1.3.5, 1.3.4]
The overwhelming majority of studies of regional climate effects on terrestrial species reveal consistent responses to warming trends, including poleward and elevational range shifts of flora and fauna. Responses of terrestrial species to warming across the Northern Hemisphere are well documented by changes in the timing of growth stages (i.e., phenological changes), especially the earlier onset of spring events, migration, and lengthening of the growing season. Changes in abundance of certain species, including limited evidence of a few local disappearances, and changes in community composition over the last few decades have been attributed to climate change (very high confidence). [ 1.3.5 ]
Many observed changes in phenology and distribution of marine species have been associated with rising water temperatures, as well as other climate-driven changes in salinity, oxygen levels, and circulation. For example, plankton has moved poleward by 10° latitude over a period of four decades in the North Atlantic. While there is increasing evidence for climate change impacts on coral reefs, separating the impacts of climate-related stresses from other stresses (e.g., over-fishing and pollution) is difficult. Warming of lakes and rivers is affecting abundance and productivity, community composition, phenology, distribution and migration of freshwater species (high confidence). [ 1.3.4 ]
Although responses to recent climate changes in human systems are difficult to identify due to multiple non-climate driving forces and the presence of adaptation, effects have been detected in forestry and a few agricultural systems [ 1.3.6 ]. Changes in several aspects of the human health system have been related to recent warming [ 1.3.7 ]. Adaptation to recent warming is beginning to be systematically documented (medium confidence) [ 1.3.9 ].
In comparison with other factors, recent warming has been of limited consequence in agriculture and forestry. A significant advance in phenology, however, has been observed for agriculture and forestry in large parts of the Northern Hemisphere, with limited responses in crop management. The lengthening of the growing season has contributed to an observed increase in forest productivity in many regions, while warmer and drier conditions are partly responsible for reduced forest productivity, increased forest fires and pests in North America and the Mediterranean Basin. Both agriculture and forestry have shown vulnerability to recent trends in heatwaves, droughts and floods (medium confidence). [ 1.3.6 ]
While there have been few studies of observed health effects related to recent warming, an increase in high temperature extremes has been associated with excess mortality in Europe, which has prompted adaptation measures. There is emerging evidence of changes in the distribution of some human disease vectors in parts of Europe. Earlier onset and increases in the seasonal production of allergenic pollen have occurred in mid- and high latitudes in the Northern Hemisphere (medium confidence). [ 1.3.7 ]
Changes in socio-economic activities and modes of human response to climate change, including warming, are just beginning to be systematically documented. In regions of snow, ice and frozen ground, responses by indigenous groups relate to changes in the migration patterns, health, and range of animals and plants on which they depend for their livelihood and cultural identity. Responses vary by community and are dictated by particular histories, perceptions of change and range, and the viability of options available to groups (medium confidence). [ 1.3.9 ]
While there is now significant evidence of observed changes in natural systems in every continent, including Antarctica, as well as from most oceans, the majority of studies come from mid- and high latitudes in the Northern Hemisphere. Documentation of observed changes in tropical regions and the Southern Hemisphere is sparse. [ 1.5 ]
1.1 Introduction
The IPCC Working Group II Third Assessment Report (WGII TAR) found evidence that recent regional climate changes, particularly temperature increases, have already affected many physical and biological systems, and also preliminary evidence for effects in human systems ( IPCC, 2001a [NPR] ). This chapter focuses on studies since the TAR that analyse significant changes in physical, biological and human systems related to observed regional climate change. The studies are assessed with regard to current functional understanding of responses to climate change and to factors that may confound such relationships, such as land-use change, urbanisation and pollution. The chapter considers larger-scale aggregation of observed changes (across systems and geographical regions) and whether the observed changes may be related to anthropogenic climate forcing. Cases where there is evidence of climate change without evidence of accompanying changes in natural and managed systems are evaluated for insight into time-lag effects, resilience and vulnerability. Managed systems are defined as systems with substantial human inputs, such as agriculture and human health. The chapter assesses whether responses to recent warming are present in a broad range of systems and across varied geographical regions.
1.1.1 Scope and goals of the chapter
The aim of this chapter is to assess studies of observed changes in natural and managed systems related to recent regional climate change, particularly temperature rise in recent decades, and to assess the aggregate changes in regard to potential influence by anthropogenic increase in greenhouse gas concentrations. Temperature rise is selected as the major climate variable because it has a strong and widespread documented signal in recent decades, demonstrates an anthropogenic signal, and has an important influence on many physical and biological processes. Effects of changes in other climate variables related to temperature rise, such as sea-level rise and changes in runoff due to earlier snow melt, are also considered.
The chapter first reviews data sources and methods of detection of observed changes, investigating the roles of climate (including climate extremes and large-scale natural climate variability systems) and non-climate drivers of change ( Section 1.2 ). Evidence of no change, i.e., regions with documented warming trends but with little or no documentation of change in natural and managed systems, is analysed as well.
In Section 1.3 , evidence is assessed regarding recent observed changes in natural and managed systems related to regional climate changes: cryosphere (snow, ice and frozen ground – including permafrost), hydrology and water resources, coastal processes and zones, marine and freshwater biological systems, terrestrial biological systems, agriculture and forestry, human health, and disasters and hazards. Evidence regarding other socio-economic effects, including energy use and tourism, is also assessed. The term ‘response’ is used to denote processes by which natural and managed systems react to the stimuli of changing climate conditions.
In Section 1.4 , studies are surveyed that use techniques of larger-scale aggregation (i.e., synthesising studies across systems and regions), including meta-analyses and studies that relate observed changes in natural and managed systems to anthropogenic climate change. From the studies assessed in individual systems in Section 1.3 , a subset is selected that fits criteria in regard to length of study and statistically significant changes in a system related to recent changes in temperature or related climate variables, in order to assess the potential influence of anthropogenic climate forcing on observed changes in natural and managed systems.
We consider what observed changes are contributing to the study of adaptation and vulnerability (where there are relevant studies), and address data needs in Section 1.5 . There is a notable lack of geographical balance in the data and literature on observed changes in natural and managed systems, with a marked scarcity in many regions. The Supplementary Material [1] (SM) contains additional literature citations and explanatory data relevant to the chapter.
1.1.2 Summary of observed changes in the Third Assessment Report
The Working Group I (WGI) TAR described an increasing body of observations that gave a collective picture of a warming world and other changes in the climate system ( IPCC, 2001b [NPR] ). The WGII TAR documented methods of detecting observed changes in natural and managed systems, characterised the processes involved, and summarised the studies across multiple systems (see Sections 2.2, 5.2.1 and 19.1) ( IPCC, 2001a [NPR] ). In the TAR, about 60 studies considered about 500 data series in physical or biological systems.
Changes in physical systems:
- Sea ice: Arctic sea-ice extent had declined by about 10 to 15% since the 1950s. No significant trends in Antarctic sea-ice extent were apparent.
- Glaciers and permafrost: mountain glaciers were receding on all continents, and Northern Hemisphere permafrost was thawing.
- Snow cover: extent of snow cover in the Northern Hemisphere had decreased by about 10% since the late 1960s and 1970s.
- Snow melt and runoff: snowmelt and runoff had occurred increasingly earlier in Europe and western North America since the late 1940s.
- Lake and river ice: annual duration of lake- and river-ice cover in Northern Hemisphere mid- and high latitudes had been reduced by about 2 weeks and become more variable.
Changes in biological systems:
- Range: plant and animal ranges had shifted poleward and higher in elevation.
- Abundance: within the ranges of some plants and animals, population sizes had changed, increasing in some areas and declining in others.
- Phenology: timing of many life-cycle events, such as blooming, migration and insect emergence, had shifted earlier in the spring and often later in the autumn.
- Differential change: species changed at different speeds and in different directions, causing a decoupling of species interactions (e.g., predator-prey relationships).
Preliminary evidence for changes in human systems:
- Damages due to droughts and floods: changes in some socio-economic systems had been related to persistent low rainfall in the Sahelian region of Africa and to increased precipitation extremes in North America. Most of the increase in damages is due to increased wealth and exposure. However, part of the increase in losses was attributed to climate change, in particular to more frequent and intense extreme weather events in some regions.
1.2 Methods of detection and attribution of observed changes
In the TAR ( Mitchell et al., 2001 [NPR, SRC] ), detection of climate change is the process of demonstrating that an observed change is significantly different (in a statistical sense) from what can be explained by natural variability. The detection of a change, however, does not necessarily imply that its causes are understood. Similarly, attribution of climate change to anthropogenic causes involves statistical analysis and the assessment of multiple lines of evidence to demonstrate, within a pre-specified margin of error, that the observed changes are (1) unlikely to be due entirely to natural internal climate variability; (2) consistent with estimated or modelled responses to the given combination of anthropogenic and natural forcing; and (3) not consistent with alternative, physically plausible explanations of recent climate change.
Extending detection and attribution analysis to observed changes in natural and managed systems is more complex. Detection and attribution of observed changes and responses in systems to anthropogenic forcing is usually a two-stage process ( IPCC, 2003 [NPR] ). First, the observed changes in a system must be demonstrated to be associated with an observed regional climate change within a specified degree of confidence. Second, a measurable portion of the observed regional climate change, or the associated observed change in the system, must be attributed to anthropogenic causes with a similar degree of confidence.
Joint attribution involves both attribution of observed changes to regional climate change and attribution of a measurable proportion of either regional climate change or the associated observed changes in the system to anthropogenic causes, beyond natural variability. This process involves statistically linking climate change simulations from climate models with the observed responses in the natural or managed system. Confidence in joint attribution statements must be lower than the confidence in either of the individual attribution steps alone, due to the combination of two separate statistical assessments.
1.2.1 Climate and non-climate drivers of change
Both climate and non-climate drivers affect systems, making analysis of the role of climate in observed changes challenging. Non-climate drivers such as urbanisation and pollution can influence systems directly and indirectly through their effects on climate variables such as albedo and soil-moisture regimes. Socio-economic processes, including land-use change (e.g., forestry to agriculture; agriculture to urban area) and land-cover modification (e.g., ecosystem degradation or restoration) also affect multiple systems.
1.2.1.1 Climate drivers of change
Climate is a key factor determining different characteristics and distributions of natural and managed systems, including the cryosphere, hydrology and water resources, marine and freshwater biological systems, terrestrial biological systems, agriculture and forestry. For example, temperature is known to strongly influence the distribution and abundance patterns of both plants and animals, due to the physiological constraints of each species ( Parmesan and Yohe, 2003 [JoC, ARC] ; Thomas et al., 2004 [JoC, ARC] ). Dramatic changes in the distribution of plants and animals during the ice ages illustrate how climate influences the distribution of species. Equivalent effects can be observed in other systems, such as the cryosphere. Hence, changes in temperature due to climate change are expected to be one of the important drivers of change in natural and managed systems.
Many aspects of climate influence various characteristics and distributions of physical and biological systems, including temperature and precipitation, and their variability on all time-scales from days to the seasonal cycle to interannual variations. While changes in many different aspects of climate may at least partially drive changes in the systems, we focus on the role of temperature changes. This is because physical and biological responses to changing temperatures are often better understood than responses to other climate parameters, and the anthropogenic signal is easier to detect for temperature than for other parameters. Precipitation has much larger spatial and temporal variability than temperature, and it is therefore more difficult to identify the impact it has on changes in many systems. Mean temperature (including daily maximum and minimum temperature) and the seasonal cycle in temperature over relatively large spatial areas show the clearest signals of change in the observed climate ( IPCC, 2001b [NPR] ).
Large-scale climate variations, such as the Pacific Decadal Oscillation (PDO), El Niño-Southern Oscillation (ENSO) and North Atlantic Oscillation (NAO), are occurring at the same time as the global climate is changing. Consequently, many natural and managed systems are being affected by both climate change and climate variability. Hence, studies of observed changes in regions influenced by an oscillation may be able to attribute these changes to regional climate variations, but decades of data may be needed in order to separate the response to climate oscillations from that due to longer-term climate change.
Table 1.1. Direct and indirect effects of non-climate drivers.
| Non-climate driver | Examples | Direct effects on systems | Indirect effects on climate |
|---|---|---|---|
| Geological processes | Volcanic activity, earthquakes, tsunamis (e.g., Adams et al., 2003) | Lava flow, mudflows (lahars), ash fall, shock waves, coastal erosion, enhanced surface and basal melting of glaciers, rockfall and ice avalanches | Cooling from stratospheric aerosols, change in albedo |
| Land-use change | Conversion of forest to agriculture (e.g., Lepers et al., 2004) | Declines in wildlife habitat, biodiversity loss, increased soil erosion, nitrification | Change in albedo, lower evapotranspiration, altered water and heat balances (e.g., Bennett and Adams, 2004) |
| Urbanisation and transportation (e.g., Kalnay and Cai, 2003) | Ecosystem fragmentation, deterioration of air quality, increased runoff and water pollution (e.g., Turalioglu et al., 2005) | Change in albedo, urban heat island, local precipitation reduction, downwind precipitation increase, lower evaporation (e.g., Weissflog et al., 2004) | |
| Afforestation (e.g., Rudel et al., 2005) | Restoration or establishment of tree cover (e.g., Gao et al., 2002) | Change in albedo, altered water and energy balances, potential carbon sequestration | |
| Land-cover modification | Ecosystem degradation (desertification) | Reduction in ecosystem services, reduction in biomass, biodiversity loss (e.g., Nyssen et al., 2004) | Changes in microclimate (e.g., Su et al., 2004) |
| Invasive species | Tamarisk (USA), Alaska lupin (Iceland) | Reduction of biodiversity, salinisation (e.g., Lee et al., 2006) | Change in water balance (e.g., Ladenburger et al., 2006) |
| Pollution | Tropospheric ozone, toxic waste, oil spills, exhaust, pesticides increased soot emissions (e.g., Pagliosa and Barbosa, 2006) | Reduction in breeding success and biodiversity, species mortality, health impairment, enhanced melting of snow and ice (e.g., Lee et al., 2006) | Direct and indirect aerosol effects on temperature, albedo and precipitation |
1.2.1.2 Non-climate drivers of change
Non-climate drivers, such as land use, land degradation, urbanisation and pollution, affect systems directly and indirectly through their effects on climate ( Table 1.1 ). These drivers can operate either independently or in association with one another ( Lepers et al., 2004 [ARC] ). Complex feedbacks and interactions occur on all scales from local to global.
The socio-economic processes that drive land-use change include population growth, economic development, trade and migration; these processes can be observed and measured at global, regional and local scales ( (Goklany, 1996 ) ). Satellite observations demonstrate that land-use change, including that associated with the current rapid economic development in Asia and Latin America, is proceeding at an unprecedented rate ( Rindfuss et al., 2004 [JoC] ). Besides influencing albedo and evaporation, land-use changes hamper range-shift responses of species to climate change, leading to an extra loss of biodiversity ( (Opdam and Wascher, 2004 ) ). Additionally, land-use changes have been linked to changes in air quality and pollution that affect the greenhouse process itself ( Pielke et al., 2002 [NPR, MoS] ; Kalnay and Cai, 2003 [JoC] ). Land-use and land-cover change can also strongly magnify the effects of extreme climate events, e.g., heat mortality, injuries/fatalities from storms, and ecologically mediated infectious diseases ( Patz et al., 2005 [JoC, ARC] ). Intensification of land use, as well as the extent of land-use change, is also affecting the functioning of ecosystems, and hence emissions of greenhouse gases from soils, such as CO2 and methane.
There are also a large number of socio-economic factors that can influence, obscure or enhance the observed impacts of climate change and that must be taken into account when seeking a climate signal or explaining observations of impacts and even adaptations. For example, the noted effects of sea-level rise and extreme events are much greater when they occur in regions with large populations, inadequate infrastructure, or high property prices ( Pielke et al., 2003 [ARC] ). The observed impacts of climate change on agriculture are largely determined by the ability of producers to access or afford irrigation, alternate crop varieties, markets, insurance, fertilisers and agricultural extension, or to abandon agriculture for alternate livelihoods ( Eakin, 2000 [JoC] ). Demography (e.g., the elderly and the very young), poverty (e.g., malnutrition and poor living conditions), preventive technologies (e.g., pest control and immunisation), and healthcare institutions influence the impacts of climate change on humans.
1.2.2 Methods and confidence
Where long data series exist, the detection of trends or changes in system properties that are beyond natural variability has most commonly been made with regression, correlation and time-series analyses. When data exist from two (or more) discontinuous time periods, two-sample tests have frequently been employed. Testing is also done for abrupt changes and discontinuities in a data series. Regression and correlation methods are frequently used in the detection of a relationship of the observed trend with climate variables. Methods also involve studies of process-level understanding of the observed change in relation to a given regional climate change, and the examination of alternative explanations of the observed change, such as land-use change. The analysis sometimes involves comparisons of observations to climate-driven model simulations.
In many biological field studies, species within an area are not fully surveyed, nor is species selection typically based on systematic or random sampling. The selection of species is typically based on a determination of which species might provide information (e.g., on change with warming) in order to answer a particular question. The study areas, however, are often chosen at random from a particular suite of locations defined by the presence of the species being studied. This type of species selection does not provide a well-balanced means for analysing species showing no change. Exceptions are studies that rely on network data, meaning that species information is collected continuously on a large number of species over decades from the same areas; for example, change in spring green-up [2] of a number of plants recorded in phenological botanical gardens across a continent ( Menzel and Fabian, 1999 [JoC, SRC] ). Analysis of change and no-change within network data provides a check on the accuracy of the use of the indicator for global warming and the ability to check for ‘false positives’, i.e., changes observed where no significant temperature change is measured. The latter can help to elucidate the role of non-climate drivers in the observed changes.
The analysis of evidence of no change is also related to the question of publication or assessment bias. Studies are more likely to be successfully submitted and published when a significant change is found and less likely to be successful when no changes are found, with the result that the ‘no change’ cases are underrepresented in the published literature. However, in contrast to single-species in single-location studies, multiple species in a single location and single or multiple species in larger-scale studies are less likely to focus only on species showing change. The latter studies often include sub-regions with no-change; for example, no change in the number of frost days in the south-eastern USA ( (Feng and Hu, 2004 ) ), little or no change in spring onset in continental eastern Europe ( Ahas et al., 2002 [JoC, SRC] ; Schleip et al., 2006 [SRC] ), or sub-groups of species with no change ( (Butler, 2003; ) Strode, 2003 [JoC] ).
An accurate percentage of sites exhibiting ‘no change’ can be assessed reliably by large-scale network studies (see, e.g., Section 1.4.1; Menzel et al., 2006b [JoC, SRC] ) for the locations defined by the network. For investigations of a suite of processes or species at numerous locations, the reported ratio of how many species are changing over the total number of species rests on the assumptions that all species in the defined area have been examined and that species showing no change do not have a higher likelihood of being overlooked. Both multi-species network data and studies on groups of species may be used to investigate the resilience of systems and possible time-lag effects. These are important processes in the analysis of evidence of no change.
1.3 Observed changes in natural and managed systems related to regional climate changes
The following sections assess studies that have been published since the TAR of observed changes and their effects related to the cryosphere, hydrology and water resources, coastal processes and zones, freshwater and marine biological systems, terrestrial biological systems, agriculture and forestry, human health, and disasters and hazards related to regional warming. More detailed descriptions of these effects are provided in subsequent chapters of the WGII Fourth Assessment Report (AR4).
In some cases, studies published before the TAR have been included, either because they were not cited in the TAR or because they have been considered to contain relevant information. The sections describe regional climate and non-climate driving forces for the systems, assess the evidence regarding observed changes in key processes, and highlight issues regarding the absence of observed changes and conflicting evidence. An assessment of how the observed changes contribute to understanding of adaptation and vulnerability is found in Sections 1.3.9 and 1.5 .
1.3.1 Cryosphere
The cryosphere reacts sensitively to present and past climate changes. The main components of the cryosphere are mountain glaciers and ice caps, floating ice shelves and continental ice sheets, seasonal snow cover on land, frozen ground, sea ice and lake and river ice. In Chapter 4 of WGI, the changes in the cryosphere since the TAR are described in detail, including the description of climate and non-climate forcing factors and mechanisms ( Lemke et al., 2007 [NPR, SRC, 2007] ). Chapter 6 of WGI describes glacier changes in the geological past, including Holocene glacier variability ( Jansen et al., 2007 [NPR, PoC, 2007] , Box 6.3 ). Here we describe the observed effects on the environment and on human activities due to these recent cryospheric changes.
There is abundant evidence that the vast majority of the cryospheric components are undergoing generalised shrinkage in response to warming, with a few cases of growth which have been mainly linked to increased snowfall. The observed recession of glaciers ( Box 1.1 ) during the last century is larger than at any time over at least the last 5,000 years, is outside of the range of normal climate variability, and is probably induced by anthropogenic warming ( Jansen et al., 2007 [NPR, PoC, 2007] ). In the Arctic and the Antarctic, ice shelves several thousand years old have started to collapse due to warming ( Lemke et al., 2007 [NPR, SRC, 2007] ). In many cases the cryospheric shrinkage shows an increased trend in recent decades, consistent with the enhanced observed warming. Cryospheric changes are described by Lemke et al. 2007 [NPR, SRC, 2007] ), including the contribution of the cryosphere to sea-level rise. Sea-level rise is treated in Section 1.3.3 , in the regional chapters of WGII, and in WGI, Chapters 4 and 5 ( Bindoff et al., 2007 [NPR, 2007] ; Lemke et al., 2007 [NPR, SRC, 2007] ).
Box 1.1. Retreat of Chacaltaya and its effects: case study of a small disappearing glacier in Bolivia
The observed general glacier retreat in the warming tropical Andes has increased significantly in recent decades ( Francou et al., 2005 [NPR, SRC] ). Small-sized glaciers are particularly vulnerable in warmer climates, with many of them having already disappeared in several parts of the world during the last century. The Chacaltaya Glacier in Bolivia (16°S) is a typical example of a disappearing small glacier, whose area in 1940 was 0.22 km2, and which has currently reduced (in 2005 ) to less than 0.01 km2 ( Figure 1.1 ( Ramirez et al., 2001 [SRC] ; Francou et al., 2003 [JoC, SRC] ; Berger et al., 2005 [NPR, SRC] ), with current estimates showing that it may disappear completely before 2010 . In the period 1992 to 2005, the glacier suffered a loss of 90% of its surface area, and 97% of its volume of ice ( Berger et al., 2005 [NPR, SRC] ). Although, in the tropics, glacier mass balance responds sensitively to changes in precipitation and humidity (see Lemke et al., 2007 [NPR, SRC, 2007] , Section 4.5.3), the fast glacier shrinkage of Chacaltaya is consistent with an ascent of the 0°C isotherm of about 50 m/decade in the tropical Andes since the 1980 s ( Vuille et al., 2003 [JoC, MoS] ), resulting in a corresponding rise in the equilibrium line of glaciers in the region ( Coudrain et al., 2005 [SRC] ).
Ice melt from Chacaltaya Glacier, located in Choqueyapu Basin, provides part of the water resources for the nearby city of la Paz, allowing the release of water stored as ice throughout the long, dry winter season (April-September). Many basins in the tropical Andes have experienced an increase in runoff in recent decades, while precipitation has remained almost constant or has shown a tendency to decrease ( Coudrain et al., 2005 [SRC] ). This short-term increase in runoff is interpreted as the consequence of glacier retreat, but in the long term there will be a reduction in water supply as the glaciers shrink beyond a critical limit ( (Jansson et al., 2003 ) ).
Chacaltaya Glacier, with a mean altitude of 5,260 m above sea level, was the highest skiing station in the world until a very few years ago. After the accelerated shrinkage of the glacier during the 1990 s, enhanced by the warm 1997 /98 El Niño, Bolivia lost its only ski area ( Figure 1.1 ), directly affecting the development of snow sports and recreation in this part of the Andes, where glaciers are an important part of the cultural heritage.
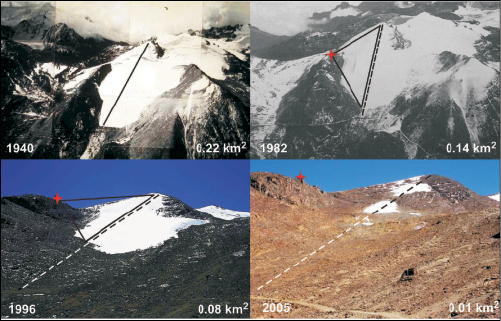
Figure 1.1. Areal extent of Chacaltaya Glacier, Bolivia, from 1940 to 2005 . By 2005, the glacier had separated into three distinct small bodies. The position of the ski hut, which did not exist in 1940, is indicated with a red cross. The ski lift, which had a length of about 800 m in 1940 and about 600 m in 1996, was normally installed during the summer months (precipitation season in the tropics) and covered a major portion of the glacier, as indicated with a continuous line. The original location of the ski lift in 1940 is indicated with a segmented line in subsequent epochs. After 2004, skiing was no longer possible. Photo credits: Francou and Vincent 2006 [NPR, SRC] and Jordan 1991 [NPR] ).
1.3.1.1 Observed effects due to changes in the cryosphere
Effects of changes in the cryosphere have been documented in relation to virtually all of the cryospheric components, with robust evidence that it is, in general, a response to reduction of snow and ice masses due to enhanced warming.
Mountain glaciers and ice caps, ice sheets and ice shelves
Effects of changes in mountain glaciers and ice caps have been documented in runoff, changing hazard conditions ( Haeberli and Burn, 2002 [NPR, SRC] ) and ocean freshening ( Bindoff et al., 2007 [NPR, 2007] ). There is also emerging evidence of present crustal uplift in response to recent glacier melting in Alaska ( (Larsen et al., 2005 ) ). The enhanced melting of glaciers leads at first to increased river runoff and discharge peaks and an increased melt season ( (Boon et al., 2003; ) Hock, 2005 [MoS] ; Hock et al., 2005 [MoS] ; Juen et al., 2007 [NPR, SRC, 2007] ), while in the longer time-frame (decadal to century scale), glacier wasting should be amplified by positive feedback mechanisms and glacier runoff is expected to decrease ( (Jansson et al., 2003 ) ). Evidence for increased runoff in recent decades due to enhanced glacier melt has already been detected in the tropical Andes and in the Alps. As glaciers disappear, the records preserved in the firn [3] and ice layers are destroyed and disappear due to percolation of melt water and mixing of chemical species and stable isotopes ( Table 1.2 ).
Table 1.2. Selected observed effects due to changes in the cryosphere produced by warming.
| Environmental factor | Observed changes | Time period | Location | Selected references |
|---|---|---|---|---|
| Glacial lake size | Increase from 0.23 km2 to 1.65 km2 | 1957-1997 | Lake Tsho Rolpa, Nepal Himalayas | Agrawala et al., 2005 |
| Glacial lake outburst floods (GLOFs) | Frequency increase from 0.38 events/year in 1950s to 0.54 events/year in 1990s | 1934-1998 | Himalayas of Nepal, Bhutan and Tibet | Richardson and Reynolds, 2000 |
| Obliteration of firn/ice core record | Percolation, loss of palaeoclimate record | 1976-2000 | Quelccaya ice cap, Peru | Thompson et al., 2003 |
| Reduction in mountain ice | Loss of ice climbs | 1900-2000 | Andes, Alps, Africa | Schwörer, 1997; Bowen, 2002 |
| Travel days of vehicles for oil exploration on frozen roads | Decrease from 220 to 130 days | 1971-2003 | Alaskan tundra | ACIA, 2005 |
| Decreased snow in ski areas at low altitudes | Decrease in number of ski areas from 58 to 17 | 1975-2002 | New Hampshire, north-eastern USA | Hamilton, 2003b |
| 50% (15%) decrease in snow depth at an elevation of 440 m (2,220 m) | 1975-1999 | Swiss Alps | Laternser and Schneebeli, 2003 | |
| 50% decrease of 1 Dec–30 April snow depth at 1,320 m elevation | 1960-2005 | Massifs de Chartreuse, Col de Porte, French Pre-Alps, | Francou and Vincent, 2006 | |
| Increase in elevation of starting point of ski lifts from 1,400 to 2,935 m | 1950-1987 | Central Andes, Chile | Casassa et al., 2003 | |
|
Increased rockfall after the 2003 summer heatwave |
Active layer deepening from 30% to 100% of the depth measured before the heatwave | June-August 2003 | Swiss Alps | Noetzli et al., 2003; Gruber et al., 2004; Schär et al., 2004 |
The formation of large lakes is occurring as glaciers retreat from prominent Little Ice Age (LIA) moraines in several steep mountain ranges, including the Himalayas ( (Yamada, 1998; ) Mool et al., 2001 [NPR] ; Richardsonand ( Reynolds, 2000 ) ), the Andes ( Ames et al., 1989 [NPR] ; Kaser and Osmaston, 2002 [NPR, SRC] ) and the Alps ( Haeberli et al., 2001 [SRC] ; Huggel et al., 2004 [SRC] ;( Kaab et al., 2005 ) ) ( Table 1.2 ). Thawing of buried ice also threatens to destabilise the LIA moraines (e.g., Kaser and Osmaston, 2002 [NPR, SRC] ). These lakes thus have a high potential for glacial lake outburst floods (GLOFs). Governmental institutions in the respective countries have undertaken extensive safety work, and several of the lakes are now either solidly dammed or drained, but continued vigilance is needed since many tens of potentially dangerous glacial lakes still exist in the Himalayas ( (Yamada, 1998 ) ) and the Andes ( (Ames, 1998 ) ), together with several more in other mountain ranges of the world. The temporary increase in glacier melt can also produce enhanced GLOFs, as has been reported in Chile ( Peña and Escobar, 1985 [NPR] ), although these have not been linked with any long-term climate trends.
Enhanced colonisation of plants and animals in deglaciated terrain is a direct effect of glacier and snow retreat (e.g., Jones and Henry, 2003 [ARC] ). Although changes due to other causes such as introduction by human activities, increased UV radiation, contaminants and habitat loss might be important (e.g., ( Frenot et al., 2005 ) ), ‘greening’ has been reported in relation to warming in the Arctic and also in the Antarctic Peninsula. Tundra areas in the northern circumpolar high latitudes derived from a 22-year satellite record show greening trends, while forest areas show declines in photosynthetic activity ( (Bunn and Goetz, 2006 ) ). Ice-water microbial habitats have contracted in the Canadian High Arctic ( (Vincent et al., 2001 ) ).
Glacier retreat causes striking changes in the landscape, which has affected living conditions and local tourism in many mountain regions around the world ( Watson and Haeberli, 2004 [SRC] ; Mölg et al., 2005 [NPR, SRC] ). Warming produces an enhanced spring-summer melting of glaciers, particularly in areas of ablation, with a corresponding loss of seasonal snow cover that results in an increased exposure of surface crevasses, which can in turn affect, for example, snow runway operations, as has been reported in the Antarctic Peninsula ( Rivera et al., 2005 [SRC] ). The retreat, enhanced flow and collapse of glaciers, ice streams and ice shelves can lead to increased production of iceberg calving, which can in turn affect sea navigation, although no evidence for this exists as yet.
Snow cover
Spring peak river flows have been occurring 1-2 weeks earlier during the last 65 years in North America and northern Eurasia. There is also evidence for an increase in winter base flow in northern Eurasia and North America. These changes in river runoff are described in detail in Section 1.3.2 and Table 1.3 . There is also a measured trend towards less snow at low altitudes, which is affecting skiing areas ( Table 1.2 ).
Frozen ground
Degradation of seasonally frozen ground and permafrost, and an increase in active-layer thickness, should result in an increased importance of surface water ( McNamara et al., 1999 [MoS] ), with an initial but temporary phase of lake expansion due to melting, followed by their disappearance due to draining within the permafrost, as has been detected in Alaska ( (Yoshikawa and Hinzman, 2003 ) ) and in Siberia ( Smith et al., 2005 [Ambiguous] ).
Permafrost and frozen ground degradation are resulting in an increased areal extent of wetlands in the Arctic, with an associated ‘greening’, i.e., plant colonisation (see above). Wetland changes also affect the fauna. Permafrost degradation and wetland increase might produce an increased release of carbon in the form of methane to the atmosphere in the future (e.g., Lawrence and Slater, 2005 [JoC, MoS] ; Zimov et al., 2006 [JoC] ), but this has not been documented.
The observed permafrost warming and degradation, together with an increasing depth of the active layer, should result in mechanical weakening of the ground, and ground subsidence and formation of thermokarst will have a weakening effect on existing infrastructure such as buildings, roads, airfields and pipelines ( (Couture et al., 2000; ) Nelson, 2003 [JoC, ARC] ), but there is no solid evidence for this yet. There is evidence for a decrease in potential travel days of vehicles over frozen roads in Alaska ( Table 1.2 ). Permafrost melting has produced increased coastal erosion in the Arctic (e.g., ( Beaulieu and Allard, 2003 ) ); this is detailed in Section 1.3.3 .
Thawing and deepening of the active layer in high-mountain areas can produce slope instability and rock falls ( Watson and Haeberli, 2004 [SRC] ), which in turn can trigger outburst floods ( Casassa and Marangunic, 1993 [SRC] ;( Carey, 2005 ) ), but there is no evidence for trends. A reported case linked to warming is the exceptional rock-fall activity in the Alps during the 2003 summer heatwave ( Table 1.2 ).
Sea ice
Nutritional stresses related to longer ice-free seasons in the Beaufort Sea may be inducing declining survival rates, smaller size, and cannibalism among polar bears ( (Amstrup et al., 2006; ) Regehr et al., 2006 [NPR] ). Polar bears are entirely dependent on sea ice as a platform to access the marine mammals that provide their nutritional needs ( Amstrup, 2003 [NPR] ). Reduced sea ice in the Arctic will probably result in increased navigation, partial evidence of which has already been found ( Eagles, 2004 [NPR] ), and possibly also a rise in offshore oil operations, with positive effects such as enhanced trade, and negative ones such as increased pollution ( Chapter 15 ACIA, 2005 [NPR] ), but there are no quantitative data to support this.
Increased navigability in the Arctic should also raise issues of water sovereignty versus international access for shipping through the North-west and North-east Passages. Previously uncharted islands and seamounts have been discovered due to a reduction in sea ice cover ( Mohr and Forsberg, 2002 [JoC] ), which can be relevant for territorial and ocean claims.
Ocean freshening, circulation and ecosystems
There is evidence for freshening in the North Atlantic and in the Ross Sea, which is probably linked to glacier melt ( Bindoff et al., 2007 [NPR, 2007] ). There is no significant evidence of changes in the Meridional Overturning Circulation at high latitudes in the North Atlantic Ocean or in the Southern Ocean, although important changes in interannual to decadal scales have been observed in the North Atlantic ( Bindoff et al., 2007 [NPR, 2007] ). Ocean ecosystem impacts such as a reduction of krill biomass and an increase in salps in Antarctica, decline of marine algae in the Arctic due to their replacement by freshwater species, and impacts on Arctic mammals, are described in Section 1.3.4.2 .
Lake and river ice
Seasonal and multi-annual variations in lake and river ice are relevant in terms of freshwater hydrology and for human activities such as winter transportation, bridge and pipeline crossings, but no quantitative evidence of observed effects exists yet. Shortening of the freezing period of lake and river ice by an average of 12 days during the last 150 years ( Lemke et al., 2007 [NPR, SRC, 2007] ) results in a corresponding reduction in skating activities in the Northern Hemisphere. In Europe there is some evidence for a reduction in ice-jam floods due to reduced freshwater freezing during the last century ( Svensson et al., 2006 [NPR, ARC] ). Enhanced melt conditions could also result in significant ice jamming due to increased break-up events, which can, in turn, result in severe flooding ( Prowse and Beltaos, 2002 [ARC] ), although there is a lack of scientific evidence that this is already happening.
Changes in lake thermal structure and quality/quantity of under-ice habitation in lakes have been reported, as well as changes in suspended particles and chemical composition (see Section 1.3.2 ). Earlier ice-out dates can have relevant effects on lake and river ecology, while changes in river-ice dynamics may also have ecological effects (see Section 1.3.4 ).
Table 1.3. Observed changes in runoff/streamflow, lake levels and floods/droughts.
| Environmental factor | Observed changes | Time period | Location | Selected references |
|---|---|---|---|---|
| Runoff/ streamflow | Annual increase of 5%, winter increase of 25 to 90%, increase in winter base flow due to increased melt and thawing permafrost | 1935-1999 | Arctic Drainage Basin: Ob, Lena, Yenisey, Mackenzie | Lammers et al., 2001; Serreze et al., 2002; Yang et al., 2002 |
| 1 to 2 week earlier peak streamflow due to earlier warming-driven snow melt | 1936-2000 | Western North America, New England, Canada, northern Eurasia | Cayan et al., 2001; Beltaos, 2002; Stone et al., 2002; Yang et al., 2002; Hodgkins et al., 2003; Ye and Ellison, 2003; Dery and Wood, 2005; McCabe and Clark, 2005; Regonda et al., 2005 | |
| Runoff increase in glacial basins in Cordillera Blanca, Peru | 23% increase in glacial melt 143% increase 169% increase | 2001-4 vs. 1998-9 1953-1997 2000-2004 | Yanamarey Glacier catchment Llanganuco catchment Artesonraju catchment | Mark et al., 2005 Pouyaud et al., 2005 Pouyaud et al., 2005 |
| Floods | Increasing catastrophic floods of frequency (0.5 to 1%) due to earlier break-up of river-ice and heavy rain | Last years | Russian Arctic rivers | Smith, 2000; Buzin et al., 2004; Frolov et al., 2005 |
| Droughts | 29% decrease in annual maximum daily streamflow due to temperature rise and increased evaporation with no change in precipitation | 1847-1996 | Southern Canada | Zhang et al., 2001 |
| Due to dry and unusually warm summers related to warming of western tropical Pacific and Indian Oceans in recent years | 1998-2004 | Western USA | Andreadis et al., 2005; Pagano and Garen, 2005 | |
| Water temperature | 0.1 to 1.5°C increase in lakes | 40 years | Europe, North America, Asia (100 stations) | Livingstone and Dokulil, 2001; Ozaki et al., 2003; Arhonditsis et al., 2004; Dabrowski et al., 2004; Hari et al., 2006 |
| 0.2 to 0.7°C increase (deep water) in lakes | 100 years | East Africa (6 stations) | Hecky et al., 1994; O’Reilly et al., 2003; Lorke et al., 2004; Vollmer et al., 2005 | |
| Water chemistry | Decreased nutrients from increased stratification or longer growing period in lakes and rivers | 100 years | North America, Europe, Eastern Europe, East Africa (8 stations) | Hambright et al., 1994; Adrian et al., 1995; Straile et al., 2003; Shimaraev and Domysheva, 2004; O’Reilly, 2007 |
| Increased catchment weathering or internal processing in lakes and rivers. | 10-20 years | North America, Europe (88 stations) | Bodaly et al., 1993; Sommaruga-Wograth et al., 1997; Rogora et al., 2003; Vesely et al., 2003; Worrall et al., 2003; Karst-Riddoch et al., 2005 |
1.3.1.2 Summary of cryosphere
There is abundant and significant evidence that most of the cryospheric components in polar regions and in mountains are undergoing generalised shrinkage in response to warming, and that their effects in the environment and in human activities are already detectable. This agrees with the results presented in Chapter 9 of WGI ( Hegerl et al., 2007 [NPR, ARC, 2007] ), which concludes that the observed reductions in Arctic sea ice extent, decreasing trend in global snow cover, and widespread retreat and melting of glaciers are inconsistent with simulated internal variability, and consistent with the simulated response to anthropogenic gases. The observed effects of cryosphere reduction include modification of river regimes due to enhanced glacial melt, snowmelt advance and enhanced winter base flow; formation of thermokarst terrain and disappearance of surface lakes in thawing permafrost; decrease in potential travel days of vehicles over frozen roads in the Arctic; enhanced potential for glacier hazards and slope instability due to mechanical weakening driven by ice and permafrost melting; regional ocean freshening; sea-level rise due to glacier and ice sheet shrinkage; biotic colonisation and faunal changes in deglaciated terrain; changes in freshwater and marine ecosystems affected by lake-ice and sea-ice reduction; changes in livelihoods; reduced tourism activities related to skiing, ice climbing and scenic activities in cryospheric areas affected by degradation; and increased ease of ship transportation in the Arctic.
This section focuses on the relationship of runoff, lake levels, groundwater, floods and droughts, and water quality, with observed climate variability, climate trends, and land-use and land-cover changes reported since the TAR. The time period under consideration is primarily 1975 to 2005, with many studies extending to earlier decades. Observed changes in precipitation and aspects of surface hydrology are described in more detail by Trenberth et al. 2007 [NPR, PoC, 2007] ), Section 3.3.
1.3.2 Hydrology and water resources
1.3.2.1 Changes in surface and groundwater systems
Since the TAR there have been many studies related to trends in river flows during the 20th century at scales ranging from catchment to global. Some of these studies have detected significant trends in some indicators of river flow, and some have demonstrated statistically significant links with trends in temperature or precipitation; but no globally homogeneous trend has been reported. Many studies, however, have found no trends, or have been unable to separate the effects of variations in temperature and precipitation from the effects of human interventions in the catchment, such as land-use change and reservoir construction. Variation in river flows from year to year is also very strongly influenced in some regions by large-scale atmospheric circulation patterns associated with ENSO, NAO and other variability systems that operate at within-decadal and multi-decadal time-scales.
At the global scale, there is evidence of a broadly coherent pattern of change in annual runoff, with some regions experiencing an increase at higher latitudes and a decrease in parts of West Africa, southern Europe and southern Latin America ( Milly et al., 2005 [JoC] ( Labat et al. 2004 ) ) claimed a 4% increase in global total runoff per 1°C rise in temperature during the 20th century, with regional variation around this trend, but this has been challenged ( (Legates et al., 2005 ) ) due to the effects of non-climatic drivers on runoff and bias due to the small number of data points. Gedney et al., 2006 [JoC, ARC] ) gave the first tentative evidence that CO2 forcing leads to increases in runoff due to the ecophysiological controls of CO2, although other evidence for such a relationship is difficult to find. The methodology used to search for trends can also influence results, since omitting the effects of cross-correlation between river catchments can lead to an overestimation of the number of catchments showing significant trends ( (Douglas et al., 2000 ) ). Runoff studies that show no trends are listed in the Chapter 1 Supplementary Material (SM).
Runoff in snow basins
There is abundant evidence for an earlier occurrence of spring peak river flows and an increase in winter base flow in basins with important seasonal snow cover in North America and northern Eurasia, in agreement with local and regional climate warming in these areas ( Table 1.3 ). The early spring shift in runoff leads to a shift in peak river runoff away from summer and autumn, which are normally the seasons with the highest water demand, resulting in consequences for water availability (see Chapter 3 ). See Table SM1.1a for additional changes in runoff/streamflow.
Groundwater
Groundwater in shallow aquifers is part of the hydrological cycle and is affected by climate variability and change through recharge processes ( Chen et al., 2002 [MoS] ), as well as by human interventions in many locations ( Petheram et al., 2001 [MoS] ). In the Upper Carbonate Aquifer near Winnipeg, Canada, shallow well hydrographs show no obvious trends, but exhibit variations of 3 to 4 years correlated with changes in annual temperature and precipitation ( Ferguson and George, 2003 [MoS] ).
Lakes
At present, no globally consistent trend in lake levels has been found. While some lake levels have risen in Mongolia and China (Xinjiang) in response to increased snow and ice melt, other lake levels in China (Qinghai), Australia, Africa (Zimbabwe, Zambia and Malawi), North America (North Dakota) and Europe (central Italy) have declined due to the combined effects of drought, warming and human activities. Within permafrost areas in the Arctic, recent warming has resulted in the temporary formation of lakes due to the onset of melting, which then drain rapidly due to permafrost degradation (e.g., Smith et al., 2005 [Ambiguous] ). A similar effect has been reported for a lake formed over an Arctic ice shelf (i.e., an epishelf lake), which disappeared when the ice shelf collapsed ( Mueller et al., 2003 [JoC] ). Permafrost and epishelf lakes are treated in detail by le Treut et al. 2007 [NPR, 2007] ). Observed trends in lake levels are listed in Table SM1.1b.
1.3.2.2 Floods and droughts
Documented trends in floods show no evidence for a globally widespread change. Although Milly et al. 2002 [JoC] ) identified an apparent increase in the frequency of ‘large’ floods (return period >100 years) across much of the globe from the analysis of data from large river basins, subsequent studies have provided less widespread evidence. Kundzewicz et al. 2005 [ARC] ) found increases (in 27 cases) and decreases (in 31 cases) and no trend in the remaining 137 cases of the 195 catchments examined worldwide. Table 1.3 shows results of selected changes in runoff/streamflow, lake levels and floods/droughts. Other examples of changes in floods and droughts may be found in Table SM1.2.
Globally, very dry areas (Palmer Drought Severity Index, PDSI ² -3.0) have more than doubled since the 1970 s due to a combination of ENSO events and surface warming, while very wet areas (PDSI ³ +3.0) declined by about 5%, with precipitation as the major contributing factor during the early 1980 s and temperature more important thereafter ( Dai et al., 2004 [PoC, JoC] ). The areas of increasing wetness include the Northern Hemisphere high latitudes and equatorial regions. However, the use of PDSI is limited by its lack of effectiveness in tropical regions. Table 1.3 shows the trend in droughts in some regions. Documented trends in severe droughts and heavy rains ( Trenberth et al., 2007 [NPR, PoC, 2007] , Section 3.8.2) show that hydrological conditions are becoming more intense in some regions, consistent with other findings ( (Huntington, 2006 ) ).
1.3.2.3 Changes in physical and chemical aspects of lakes and rivers
Changes in thermal structure and chemistry have been documented in many parts of the world in recent decades.
Thermal structure
Higher water temperatures have been reported in lakes in response to warmer conditions ( Table 1.3 ) (see Table SM1.3 for additional changes in physical water properties). Shorter periods of ice cover and decreases in river- and lake-ice thickness are treated in Section 1.3.1 and le Treut et al. 2007 [NPR, 2007] ). Phytoplankton dynamics and primary productivity have also been altered in conjunction with changes in lake physics (see Section 1.3.4.4 ; Figure 1.2 ; Table 1.6 ). Since the 1960 s, surface water temperatures have warmed by 0.2 to 2°C in lakes and rivers in Europe, North America and Asia. Along with warming surface waters, deep-water temperatures (which reflect long-term trends) of the large East African lakes (Edward, Albert, Kivu, Victoria, Tanganyika and Malawi) have warmed by 0.2 to 0.7°C since the early 1900 s. Increased water temperature and longer ice-free seasons influence the thermal stratification and internal hydrodynamics of lakes. In warmer years, surface water temperatures are higher, evaporative water loss increases, summer stratification occurs earlier in the season, and thermoclines become shallower. In several lakes in Europe and North America, the stratified period has advanced by up to 20 days and lengthened by 2 to 3 weeks, with increased thermal stability.
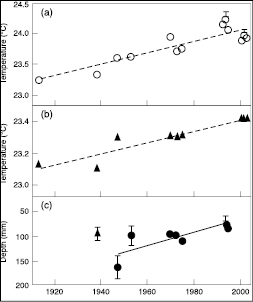
Figure 1.2. Historical and recent measurements from Lake Tanganyika, East Africa: (a) upper mixed layer (surface water) temperatures; (b) deep-water (600 m) temperatures; (c) depth of the upper mixed layer. Triangles represent data collected by a different method. Error bars represent standard deviations. Reprinted by permission from Macmillan Publishers Ltd. [Nature] ( O’Reilly et al., 2003 [JoC, SRC] ), copyright 2003 .
Chemistry
Increased stratification reduces water movement across the thermocline, inhibiting the upwelling and mixing that provide essential nutrients to the food web. There have been decreases in nutrients in the surface water and corresponding increases in deep-water concentrations of European and East African lakes because of reduced upwelling due to greater thermal stability. Many lakes and rivers have increased concentrations of sulphates, base cations and silica, and greater alkalinity and conductivity related to increased weathering of silicates, calcium and magnesium sulphates, or carbonates, in their catchment. In contrast, when warmer temperatures enhanced vegetative growth and soil development in some high-alpine ecosystems, alkalinity decreased because of increased organic-acid inputs ( (Karst-Riddoch et al., 2005 ) ). Glacial melting increased the input of organochlorines (which had been atmospherically transported to and stored in the glacier) to a sub-alpine lake in Canada ( (Blais et al., 2001 ) ).
Increased temperature also affects in-lake chemical processes ( Table 1.3 ) (also see Table SM1.3 for additional observed changes in chemical water properties). There have been decreases in dissolved inorganic nitrogen from greater phytoplankton productivity ( Sommaruga-Wograth et al., 1997 [JoC] ;( Rogora et al., 2003 ) ) and greater in-lake alkalinity generation and increases in pH in soft-water lakes ( Psenner and Schmidt, 1992 [JoC] ). Decreased solubility from higher temperatures significantly contributed to 11 to 13% of the decrease in aluminium concentration ( (Vesely et al., 2003 ) ), whereas lakes that had warmer water temperatures had increased mercury methylation and higher mercury levels in fish ( (Bodaly et al., 1993 ) ). A decrease in silicon content related to regional warming has been documented in Lake Baikal, Russia. River water-quality data from 27 rivers in Japan also suggest a deterioration in both chemical and biological features due to increases in air temperature.
1.3.2.4 Summary of hydrology and water resources
Changes in river discharge, as well as in droughts and heavy rains in some regions, indicate that hydrological conditions have become more intense. Significant trends in floods and in evaporation and evapotranspiration have not been detected globally. Some local trends in reduced groundwater and lake levels have been reported, but these are likely to be due to human activities rather than climate change. Climate-change signals related to increasing runoff and streamflow have been observed over the last century in many regions, particularly in basins fed by glaciers, permafrost and snow melt. Evidence includes increases in average runoff of Arctic rivers in Eurasia, which has been at least partly correlated with climate warming, and earlier spring snow melt and increase in winter base flow in North America and Eurasia due to enhanced seasonal snow melt associated with climate warming. There are also indications of intensified droughts in drier regions. Lake formation and their subsequent disappearance in permafrost have been reported in the Arctic. Freshwater lakes and rivers are experiencing increased water temperatures and changes in water chemistry. Surface and deep lake waters are warming, with advances and lengthening of periods of thermal stability in some cases associated with physical and chemical changes such as increases in salinity and suspended solids, and a decrease in nutrient content.
Global sea level has been rising at a rate of about 1.7 to 1.8 mm/yr over the last century, with an increased rate of about 3 mm/yr during the last decade ( Church et al., 2004 [JoC, MoS] ; Holgate and Woodworth, 2004 [JoC] ; Church and White, 2006 [JoC] ; Bindoff et al., 2007 [NPR, 2007] , Section 5.5 ).
1.3.3 Coastal processes and zones
Many coastal regions are already experiencing the effects of relative (local) sea-level rise, from a combination of climate-induced sea-level rise, geological and anthropogenic-induced land subsidence, and other local factors. A major challenge, however, is to separate the different meteorological, oceanographic, geophysical and anthropogenic processes affecting the shoreline in order to identify and isolate the contribution of global warming. An unambiguous attribution of current sea-level rise as a primary driver of shoreline change is difficult to determine at present.
1.3.3.1 Changes in coastal geomorphology
Sea-level rise over the last 100 to 150 years is probably contributing to coastal erosion in many places, such as the East Coast of the USA, where 75% of the shoreline removed from the influence of spits, tidal inlets and engineering structures is eroding ( (Leatherman et al., 2000; ) Daniel, 2001 [JoC] ; Zhang et al., 2004 [JoC] ) ( Table 1.4 ; see Table SM1.4 for observations of changes in storm surges, flood height and areas, and waves). Over the last century, 67% of the eastern coastline of the UK has retreated landward of the low-water mark ( (Taylor et al., 2004 ) ).
Table 1.4. Changes in coastal processes.
| Type of change | Observed changes | Period | Location | References |
|---|---|---|---|---|
| Shoreline erosion | 75% of shoreline, uninfluenced by inlets and structures, is eroding | mid-1800s to 2000 | East Coast USA | Zhang et al., 2004 |
| Shoreline retreat, 0.61 m/yr | 1855-2002 | Louisiana, USA | Penland et al., 2005 | |
| Shoreline retreat, 0.94 m/yr | 1988-2002 | |||
| Beach erosion prevalent due to sea-level rise, mangrove clearance | 1960s-1990s | Fiji | Mimura and Nunn, 1998 | |
| Beach erosion due to coral bleaching, mangrove clearance, sand mining, structures | 1950s-2000 | Tropics: SE Asia, Indian Ocean, Australia, Barbados | Wong, 2003 | |
| 19% of studied shoreline is retreating, in spite of land uplift, due to thawing of permafrost | 1950-1995 | Manitounuk Strait, Canada | Beaulieu and Allard, 2003 | |
| Shoreline erosion, recent acceleration | Pre-1990s to present | Estuary and Gulf of St. Lawrence, Canada | Bernatchez and Dubois, 2004; Forbes et al., 2004 | |
| Increased thermokarst erosion due to climate warming | 1970-2000 rela-tive to 1954-1970 | Arctic Ocean, Beaufort Sea coasts, Canada | Lantuit and Pollard, 2003 | |
| Beach erosion due to dams across the Nile and reduced river floods due to precipitation changes | Late 20th century | Alexandria, Egypt | Frihy et al., 1996 | |
| Coastal erosion | 1843-present | UK coastline | Taylor et al., 2004 | |
| Wetland changes | About 1,700 ha of degraded marshes became open water; non-degraded marshes decreased by 1,200 ha | 1938-1989 | Chesapeake Bay, USA | Kearney et al., 2002 |
| Decreases in salt marsh area due to regional sea-level rise and human impacts | 1920s-1999 | Long Island, NY; Connecticut, USA | Hartig et al., 2002; Hartig and Gornitz, 2004 | |
| Salt marshes keep up with sea-level rise with sufficient sediment supply | 1880-2000 | Normandy, France | Haslett et al., 2003 | |
| Landward migration of cordgrass (Spartina alterniflora) due to sea-level rise and excess nitrogen | 1995-1999; late 20th century | Rhode Island, USA | Donnelly and Bertness 2001; Bertness et al., 2002 | |
| Decrease from 12,000 to 4,000 ha, from land reclamation, wave-induced erosion and insufficient sediment | 1919-2000 | Venice, Italy | Day et al., 2005 | |
| Seaward-prograding mudflats replacing sandy beaches, due to increased dredged sediment supply | 1897-1999 | Queensland coast, Australia | Wolanski et al., 2002 | |
| Wetland losses due to sea-level rise, land reclamation, changes in wind/wave energy, tidal dynamics | 1850s-1990s | Greater Thames Estuary, UK | van der Wal and Pye, 2004 | |
| Decreased rates of deltaic wetland progradation due to reduced sediment supply from dam construction | 1960s-2003 | Yangtze River Delta, Peoples Republic of China | Yang et al., 2005 | |
| Coastal vegetation changes | Grassy marshes replaced by mangrove due to sea-level rise, water table changes | 1940-1994 | South-east Florida, USA | Ross et al., 2000 |
| Mangrove encroachment into estuarine wetlands due to changing water levels, increased nutrient load, and salt-marsh compaction during drought | 1940s-1990s | South-east Australia | Saintilan and Williams,1999; Rogers et al., 2006 |
In addition to sea-level change, coastal erosion is driven by other natural factors such as wave energy, sediment supply, or local land subsidence ( Stive, 2004 [JoC] ). In Louisiana, land subsidence has led to high average rates of shoreline retreat (averaging 0.61 m/yr between 1855 and 2002, and increasing to 0.94 m/yr since 1988 ( (Penland et al., 2005 ) ); further erosion occurred after Hurricanes Katrina and Rita in August 2005 . These two hurricanes washed away an estimated 562 km2 of coastal wetlands in Louisiana ( USGS, 2006 [NPR] ). Climate variability also affects shoreline processes, as documented by shoreline displacement in Estonia associated with increasing severe storms and high surge levels, milder winters, and reduced sea-ice cover ( (Orviku et al., 2003 ) ). Significant sections of glacially rebounding coastlines, which normally would be accreting, are nonetheless eroding, as for example along Hudson Bay, Canada ( (Beaulieu and Allard, 2003 ) ). Reduction in sea-ice cover due to milder winters has also exacerbated coastal erosion, as in the Gulf of St. Lawrence( (Bernatchez and Dubois, 2004; ) Forbes et al., 2004 [SRC] ). Degradation and melting of permafrost due to climate warming are also contributing to the rapid retreat of Arctic coastlines in many regions, such as the Beaufort and Laptev Sea coasts ( Forbes, 2005 [NPR, SRC] ).
Anthropogenic activities have intensified beach erosion in many parts of the world, including Fiji, Trinidad and parts of tropical Asia ( Mimura and Nunn, 1998 [ARC] ;( Restrepo et al., 2002; ) Singh and Fouladi, 2003 [NPR, MoS] ;( Wong, 2003 ) ). Much of the observed erosion is associated with shoreline development, clearing of mangroves ( (Thampanya et al., 2006 ) ) and mining of beach sand and coral. Sediment starvation due to the construction of large dams upstream also contributes to coastal erosion ( Frihy et al., 1996 [JoC] ;( Chen et al., 2005b; ) ( Georgiou et al., 2005; ) ( Penland et al., 2005; ) Syvitski et al., 2005b [JoC] ;( Ericson et al., 2006 ) ). Pumping of groundwater and subsurface hydrocarbons also enhances land subsidence, thereby exacerbating coastal erosion ( Syvitski et al., 2005a [NPR, SRC] ).
1.3.3.2 Changes in coastal wetlands
In the USA, losses in coastal wetlands have been observed in Louisiana ( (Boesch et al., 1994 ) ), the mid-Atlantic region ( (Kearney et al., 2002 ) ), and in parts of New England and New York ( Hartig et al., 2002 [SRC] ; Hartig and Gornitz, 2004 [NPR, SRC] ), in spite of recent protective environmental regulations ( (Kennish, 2001 ) ). Many of these marshes have had a long history of anthropogenic modification, including dredging and filling, bulkheading and channelisation, which in turn could have contributed to sediment starvation, eutrophication and ultimately marsh submergence ( Donnelly and Bertness, 2001 [JoC] ; Bertness et al., 2002 [JoC] ). In Europe, losses have been documented in south-east England between 1973 and 1998, although the rate of loss has slowed since 1988 ( (Cooper et al., 2001 ) ); elsewhere there is evidence that not all coastal wetlands are retreating, for example in Normandy, France ( (Haslett et al., 2003 ) ).
Although natural accretion rates of mangroves generally compensate for current rates of sea-level rise, of greater concern at present are the impacts of clearance for agriculture, aquaculture (particularly shrimp), forestry and urbanisation. At least 35% of the world’s mangrove forests have been removed in the last two decades but possible sea-level rise effects were not considered ( (Valiela et al., 2001 ) ). In south-eastern Australia, mangrove encroachment inland into salt-marsh environments is probably related to anthropogenic causes and climate variability, rather than sea-level rise ( (Saintilan and Williams, 1999 ) ). Landward replacement of grassy freshwater marshes by more salt-tolerant mangroves in the south-eastern Florida Everglades since the 1940 s has been attributed to the combined effects of sea-level rise and water management, resulting in lowered watertables ( (Ross et al., 2000 ) ).
Sea-level rise can have a larger impact on wetland ecosystems when the human land-use pressure in the coastal area is large, e.g., coasts defended by dykes and urbanisation. Wetlands disappear or become smaller when human land use makes inward movement of the ecosystem impossible ( Wolters et al., 2005 [ARC] ).
1.3.3.3 Changes in storm surges, flood heights and areas, and waves
The vulnerability of the coastal zone to storm surges and waves depends on land subsidence, changes in storminess, and sea-level rise (see Supplementary Material). Along the North American East Coast, although there has been no significant long-term change in storm climatology, storm-surge impacts have increased due to regional sea-level rise ( Zhang et al., 2000 [JoC] ). The U.S. Gulf Coast is particularly vulnerable to hurricane surges due to low elevation and relative sea-level rise (up to 1 cm/yr along parts of the Louisiana coast), only part of which is climate-related ( (Penland et al., 2005 ) ). Hurricane Katrina, in August 2005, generated surges over 4 m, with catastrophic consequences ( NOAA, 2005 [NPR] ). In Venice, Italy, the frequency of surges has averaged around 2 per year since the mid- 1960 s, compared with only 0.19 surges per year between 1830 and 1930, with land subsidence, which was exacerbated by groundwater pumping between 1930 and 1970 ( Carminati et al., 2005 [NPR] ), and expanded sea-lagoon interactions (due to channel dredging) playing a greater role than global sea-level rise ( (Camuffo and Stararo, 2004 ) ). Surges have shown a slight decrease in Brittany, France, in recent decades, largely due to changes in wind patterns ( (Pirazzoli et al., 2004 ) ).
Apparent global increases in extreme high water levels since 1975 are related to mean sea-level rise and to large-scale inter-decadal climate variability ( Woodworth and Blackman, 2004 [JoC] ). Wave height increases have been documented in the north-east Atlantic Ocean ( Woolf et al., 2002 [MoS] ), along the US Pacific North-west coast ( (Allan and Komar, 2006 ) ) and in the Maldives ( Woodworth and Blackman, 2004 [JoC] ), but decreases have been found in some areas of the Mediterranean from 1958 to 2001 ( Lionello, 2005 [NPR] ; Lionello and Sanna, 2005 [JoC] ).
1.3.3.4 Summary of coastal processes and zones
In many coastal regions, particularly in subsiding regions, local sea-level rise exceeds the 20th century global trend of 1.7 to 1.8 mm/yr. Sea-level rise, enhanced wave heights, and increased intensity of storms are affecting some coastal regions distant from human modification, e.g., polar areas and barrier beaches, mainly through coastal erosion. Coastal erosion and losses of wetlands are widespread problems today, under current rates of sea-level rise, although these are largely caused by anthropogenic modification of the shoreline.
Observational changes in marine and freshwater environments associated with climate change should be considered against the background of natural variation on a variety of spatial and temporal scales. While many of the biological responses have been associated with rising temperatures, distinguishing the effects of climate change embedded in natural modes of variability such as ENSO and the NAO is challenging.
1.3.4 Marine and freshwater biological systems
The marine pelagic realm occupies 70% of the planetary surface and plays a fundamental role in modulating the global environment via climate regulation and biogeochemical cycling ( (Legendre and Rivkin, 2002 ) ). Perhaps equally important to global climate change, in terms of modifying the biology of the oceans, is the impact of anthropogenic CO2 on the pH of the oceans, which will affect the process of calcification for some marine organisms ( Feely et al., 2004 [JoC] ), but effects of this are as yet undocumented. Other driving forces of change that are operative in marine and freshwater biological systems are over-fishing and pollution from terrestrial runoff (from deforestation, agriculture and urban development) and atmospheric deposition, and human introduction of non-native species.
1.3.4.1 Changes in coral reefs
Concerns about the impacts of climate change on coral reefs centre on the effects of the recent trends in increasing acidity (via increasing CO2), storm intensity and sea surface temperatures (see Bindoff et al., 2007 [NPR, 2007] , Section 5.4.2.3; Trenberth et al., 2007 [NPR, PoC, 2007] , Sections 3.8.3 and 3.2.2).
Decreasing pH (see Chapter 4 , Box 4.4 ) leads to a decreased aragonite saturation state, one of the main physicochemical determinants of coral calcification ( Kleypas et al., 1999 [JoC] ). Although laboratory experiments have demonstrated a link between aragonite saturation state and coral growth ( (Langdon et al., 2000; ) ( Ohde and Hossain, 2004 ) ), there are currently no data relating altered coral growth in situ to increasing acidity.
Storms damage coral directly through wave action and indirectly through light attenuation by suspended sediment and abrasion by sediment and broken corals. Most studies relate to individual storm events, but a meta-analysis of data from 1977 to 2001 showed that coral cover on Caribbean reefs decreased by 17% on average in the year following a hurricane, with no evidence of recovery for at least 8 years post-impact ( Gardner et al., 2005 [SRC] ). Stronger hurricanes caused more coral loss, but the second of two successive hurricanes caused little additional damage, suggesting a greater future effect from increasing hurricane intensity rather than from increasing frequency ( Gardner et al., 2005 [SRC] ).
There is now extensive evidence of a link between coral bleaching – a whitening of corals as a result of the expulsion of symbiotic zooxanthellae (see Chapter 6 , Box 6.1 ) – and sea surface temperature anomalies ( McWilliams et al., 2005 [SRC] ). Bleaching usually occurs when temperatures exceed a ‘threshold’ of about 0.8-1°C above mean summer maximum levels for at least 4 weeks ( Hoegh-Guldberg, 1999 [MoS, ARC] ). Regional-scale bleaching events have increased in frequency since the 1980 s ( Hoegh-Guldberg, 1999 [MoS, ARC] ). In 1998, the largest bleaching event to date is estimated to have killed 16% of the world’s corals, primarily in the western Pacific and the Indian Ocean ( Wilkinson, 2004 [NPR] ). On many reefs, this mortality has led to a loss of structural complexity and shifts in reef fish species composition ( Bellwood et al., 2006 [JoC] ;( Garpe et al., 2006; ) Graham et al., 2006 [JoC, ARC] ). Corals that recover from bleaching suffer temporary reductions in growth and reproductive capacity ( Mendes and Woodley, 2002 [NPR] ), while the recovery of reefs following mortality tends to be dominated by fast-growing and bleaching-resistant coral genera ( (Arthur et al., 2005 ) ).
While there is increasing evidence for climate change impacts on coral reefs, disentangling the impacts of climate-related stresses from other stresses (e.g., over-fishing and pollution; Hughes et al., 2003b [JoC, SRC] ) is difficult. In addition, inter-decadal variation in pH ( Pelejero et al., 2005 [JoC] ), storm activity ( Goldenberg et al., 2001 [JoC] ) and sea surface temperatures ( (Mestas-Nunez and Miller, 2006 ) ) linked, for example, to the El Niño-Southern Oscillation and Pacific Decadal Oscillation, make it more complicated to discern the effect of anthropogenic climate change from natural modes of variability ( Section 1.3.4 ). An analysis of bleaching in the Caribbean indicates that 70% of the variance in geographic extent of bleaching between 1983 and 2000 could be attributed to variation in ENSO and atmospheric dust ( Gill et al., 2006 [JoC, SRC] ).
1.3.4.2 Changes in marine ecosystems
There is an accumulating body of evidence to suggest that many marine ecosystems, including managed fisheries, are responding to changes in regional climate caused predominately by warming of air and sea surface temperatures (SSTs) and to a lesser extent by modification of precipitation regimes and wind patterns ( Table 1.5 ). The biological manifestations of rising SSTs have included biogeographical, phenological, physiological and species abundance changes. The evidence collected and modelled to date indicates that rising CO2 has led to chemical changes in the ocean, which in turn have led to the oceans becoming more acidic (Royal Society, 2005 [NPR] ). Blended satellite/in situ ocean chlorophyll records indicate that global ocean annual primary production has declined by more than 6% since the early 1980 s ( Gregg et al., 2003 [JoC] ), whereas chlorophyll in the North-east Atlantic has increased since the mid- 1980 s ( Raitsos et al., 2005 [JoC, SRC] ).
Table 1.5. Examples of changes in marine ecosystems and managed fisheries.
| Key changes | Climate link | Location | References |
|---|---|---|---|
| Pelagic productivity/ zooplankton abundance/ plankton assemblages | Biological responses to regional changes in temperature, stratification, upwelling, and other hydro-climatic changes | North Atlantic | Fromentin and Planque, 1996; Reid et al., 1998; Edwards et al., 2002; Beaugrand et al., 2003; Johns et al., 2003; Richardson and Schoeman, 2004 |
| North Pacific | Roemmich and McGowan, 1995; Walther et al., 2002; Lavaniegos and Ohman, 2003; Chiba and Tadokoro, 2006 | ||
| South Atlantic | Verheye et al., 1998 | ||
| Southern Ocean | Walther et al., 2002; Atkinson et al., 2004 | ||
| Pelagic phenology | Earlier seasonal appearance due to increased temperature and trophic mismatch | North Sea | Edwards and Richardson, 2004; Greve, 2004 |
| Pelagic biogeography | Northerly movement of plankton communities due to general warming | Eastern North Atlantic | Beaugrand et al., 2002b |
| Southerly movement of boreal plankton in the western North Atlantic due to lower salinities | Western North Atlantic | Johns et al., 2001 | |
| Rocky shore/ intertidal communities | Community changes due to regional temperature changes | British Isles | Hawkins et al., 2003; Southward et al., 2005 |
| North Pacific | Sagarin et al., 1999 | ||
| Kelp forests/ macroalgae | Effect on communities and spread of warmer-water species due to increased temperatures | North Pacific | Holbrook et al., 1997 |
| Mediterranean | Walther et al., 2002 | ||
| Pathogens and invasive species | Geographical range shifts due to increased temperatures | North Atlantic | Harvell et al., 1999; Walther et al., 2002; McCallum et al., 2003 |
| Fish populations and recruitment success | Changes in populations, recruitment success, trophic interactions and migratory patterns related to regional environmental change | British Isles | Attrill and Power, 2002 |
| North Pacific | McGowan et al., 1998; Chavez et al., 2003 | ||
| North Atlantic | Walther et al., 2002; Beaugrand and Reid, 2003; Beaugrand et al., 2003; Brander et al., 2003; Drinkwater et al., 2003 | ||
| Barents Sea | Stenseth et al., 2002; Walther et al., 2002 | ||
| Mediterranean | Walther et al., 2002 | ||
| Bering Sea | Grebmeier et al., 2006 | ||
| Fish biogeography | Geographical range shifts related to temperature | NE Atlantic | Brander et al., 2003; Beare et al., 2004; Genner et al., 2004; Perry et al., 2005 |
| NW Atlantic | Rose and O’Driscoll, 2002 | ||
| Bering Sea | Grebmeier et al., 2006 | ||
| Seabirds and marine mammals | Population changes, migratory patterns, trophic interactions and phenology related to regional environmental change, ice habitat loss related to warming | North Atlantic | Walther et al., 2002; Drinkwater et al., 2003; Frederiksen et al., 2004 |
| North Pacific | McGowan et al., 1998; Hughes, 2000 | ||
| Southern Ocean | Barbraud and Weimerskirch, 2001; Walther et al., 2002; Weimerskirch et al., 2003; Forcada et al., 2006; Stirling and Parkinson, 2006 | ||
| Marine biodiversity | Regional response to general warming | North Atlantic | Beaugrand et al., 2002a |
In the Pacific and around the British Isles, researchers have found changes to the intertidal communities, where the composition has shifted significantly in response to warmer temperatures ( (Sagarin et al., 1999; ) ( Southward et al., 2005 ) ). Similar shifts were also noted in the kelp forest fish communities off the southern Californian coast and in the offshore zooplankton communities ( Roemmich and McGowan, 1995 [JoC] ;( Holbrook et al., 1997; ) ( Lavaniegos and Ohman, 2003 ) ). These changes are associated with oceanic warming and the resultant geographical movements of species with warmer water affinities. As in the North Atlantic, many long-term biological investigations in the Pacific have established links between changes in the biology and regional climate oscillations such as the ENSO and the Pacific Decadal Oscillation (PDO) ( Stenseth et al., 2002 [JoC] ). In the case of the Pacific, these biological changes are most strongly associated with El Niño events, which can cause rapid and sometimes dramatic responses to the short-term SST changes ( Hughes, 2000 [ARC] ). However, recent investigations of planktonic foraminifera from sediment cores encompassing the last 1,400 years has revealed anomalous change in the community structure over the last few decades. The study suggests that ocean warming has already exceeded the range of natural variability ( Field et al., 2006 [JoC] ). A recent major ecosystem shift in the northern Bering Sea has been attributed to regional climate warming and trends in the Arctic Oscillation ( Grebmeier et al., 2006 [JoC] ).
The progressive warming in the Southern Ocean has been associated with a decline in krill ( Atkinson et al., 2004 [JoC] ) and an associated decline in the population size of many seabirds and seals monitored on several breeding sites ( Barbraud and Weimerskirch, 2001 [JoC] ;( Weimerskirch et al., 2003 ) ). Some initial observations suggest that changes to the ice habitat via the total thickness of sea ice and its progressively earlier seasonal break-up in the Arctic and Antarctic caused by regional climate warming has had a detrimental impact on marine mammal and seabird populations ( (Forcada et al., 2005, ) 2006; ( Stirling and Parkinson, 2006 ) ).
In the North Atlantic, changes in both phytoplankton and zooplankton species and communities have been associated with Northern Hemisphere temperature (NHT) trends and variations in the NAO index. These have included changes in species distributions and abundance, the occurrence of sub-tropical species in temperate waters, changes in overall phytoplankton biomass and seasonal length, changes in the ecosystem functioning and productivity of the North Sea, shifts from cold-adapted to warm-adapted communities, phenological changes, changes in species interactions, and an increase in harmful algal blooms (HABs) ( Fromentin and Planque, 1996 [NPR] ; Reid et al., 1998 [JoC, SRC] ; Edwards et al., 2001 [SRC] , 2002, 2006; Reid and Edwards, 2001 [SRC] ;( Beaugrand et al., 2002a, ) 2003; Beaugrand and Reid, 2003 [JoC] ; Edwards and Richardson, 2004 [JoC, SRC] ; Richardson and Schoeman, 2004 [JoC] ). Over the last decade, numerous other investigations have established links between the NAO and the biology of the North Atlantic, including the benthos, fish, seabirds and whales ( Drinkwater et al., 2003 [NPR, SRC] ) and an increase in the incidence of marine diseases ( Harvell et al., 1999 [JoC] ). In the Benguela upwelling system in the South Atlantic, long-term trends in the abundance and community structure of coastal zooplankton have been related to large-scale climatic influences ( (Verheye et al., 1998 ) ).
Recent macroscale research has shown that the increase in regional sea temperatures has triggered a major reorganisation in calanoid copepod species composition and biodiversity over the whole North Atlantic Basin ( Figure 1.3 ( (Beaugrand et al., 2002a ) ). During the last 40 years there has been a northerly movement of warmer-water plankton by 10° latitude in the North-East Atlantic and a similar retreat of colder-water plankton to the north. This geographical movement is much more pronounced than any documented terrestrial study, presumably due to advective movements accelerating these processes. In terms of the marine phenological response to climate warming, many plankton taxa have been found to be moving forward in their seasonal cycles ( Edwards and Richardson, 2004 [JoC, SRC] ). In some cases, a shift in seasonal cycles of over six weeks was detected, but more importantly the response to climate warming varied between different functional groups and trophic levels, leading to a mismatch in timing between different trophic levels ( Edwards and Richardson, 2004 [JoC, SRC] ).
Figure 1.3. Long-term changes in the mean number of marine zooplankton species per association in the North Atlantic from 1960 to 1975 and from 1996 to 1999 . The number of temperate species has increased and the diversity of colder-temperate, sub-Arctic and Arctic species has decreased in the North Atlantic. The scale (0 to 1) indicates the proportion of biogeographical types of species in total assemblages of zooplankton. From Beaugrand et al., 2002b [JoC, SRC] . Reprinted with permission from AAAS.
1.3.4.3 Changes in marine fisheries
Northerly geographical range extensions or changes in the geographical distribution of fish populations have recently been documented for European Continental shelf seas and along the European Continental shelf edge ( Brander et al., 2003 [ARC] ; Beare et al., 2004 [JoC] ;( Genner et al., 2004; ) Perry et al., 2005 [JoC] ). These geographical movements have been related to regional climate warming and are predominantly associated with the northerly geographical movement of fish species (sardines, anchovies, red mullet and bass) with more southern biogeographical affinities. Northerly range extensions of pelagic fish species have also been reported for the Northern Bering Sea region related to regional climate warming ( Grebmeier et al., 2006 [JoC] ). New records have also been observed over the last decade for some Mediterranean and north-west African species on the south coast of Portugal ( Brander et al., 2003 [ARC] ). Cooling and freshening of the North-West Atlantic (e.g., in the sub-polar gyre, Labrador Sea and Labrador Current) over the last decade has had an opposite effect, with some groundfish species moving further south ( Rose and O’Driscoll, 2002 [ARC] ) in the same way as plankton (see 1.3.4.2).
Regional climate warming in the North Sea has affected cod recruitment via changes at the base of the food web ( Beaugrand et al., 2003 [Ambiguous] ). Key changes in the planktonic assemblage, significantly correlated with the warming of the North Sea over the last few decades, has resulted in a poor food environment for cod larvae, and hence an eventual decline in overall recruitment success. This is an example of how the dual pressures of over-fishing and regional climate warming have combined to negatively affect a commercially important fishery. Recent work on pelagic phenology in the North Sea has shown that plankton communities, including fish larvae, are very sensitive to regional climate warming, with the response varying between trophic levels and functional groups ( Edwards and Richardson, 2004 [JoC, SRC] ). The ability and speed with which fish and planktonic communities adapt to regional climate warming is not yet known.
1.3.4.4 Changes in lakes
Observations indicate that lakes and rivers around the world are warming, with effects on thermal structure and lake chemistry that in turn affect abundance and productivity, community composition, phenology, distribution and migration (see Section 1.3.2.3 ) (Tables 1.3 and 1.6 ).
Abundance/productivity
In high-latitude or high-altitude lakes where reduced ice cover has led to a longer growing season and warmer temperatures, many lakes are showing increased algal abundance and productivity over the past century ( Schindler et al., 1990 [JoC] ;( Hambright et al., 1994; ) ( Gajewski et al., 1997; ) ( Wolfe and Perren, 2001; ) ( Battarbee et al., 2002; ) ( Korhola et al., 2002; ) ( Karst-Riddoch et al., 2005 ) ). There have been similar increases in the abundance of zooplankton, correlated with warmer water temperatures and longer growing seasons ( (Adrian and Deneke, 1996; ) Straile and Adrian, 2000 [JoC] ;( Battarbee et al., 2002; ) ( Gerten and Adrian, 2002; ) ( Carvalho and Kirika, 2003; ) Winder and Schindler, 2004b [JoC] ;( Hampton, 2005; ) ( Schindler et al., 2005 ) ). For upper trophic levels, rapid increases in water temperature after ice break-up have enhanced fish recruitment in oligotrophic lakes ( (Nyberg et al., 2001 ) ). In contrast to these lakes, some lakes, particularly deep tropical lakes, are experiencing reduced algal abundance and declines in productivity because stronger stratification reduces upwelling of the nutrient-rich deep water ( Verburg et al., 2003 [JoC] ; O’Reilly, 2007 [NPR, SRC, 2007] ). Primary productivity in Lake Tanganyika may have decreased by up to 20% over the past 200 years ( O’Reilly et al., 2003 [JoC, SRC] ), and for the East African Rift Valley lakes, recent declines in fish abundance have been linked with climatic impacts on lake ecosystems ( O’Reilly, 2007 [NPR, SRC, 2007] ).
Community composition
Increases in the length of the ice-free growing season, greater stratification, and changes in relative nutrient availability have generated shifts in community composition. Of potential concern to human health is the increase in relative abundance of cyanobacteria, some of which can be toxic, in some freshwater ecosystems ( (Carmichael, 2001; ) ( Weyhenmeyer, 2001; ) ( Briand et al., 2004 ) ). Palaeolimnological records have shown widespread changes in phytoplankton species composition since the mid-to-late 1800 s due to climate shifts, with increases in chrysophytes and planktonic diatom species and decreases in benthic species ( (Gajewski et al., 1997; ) ( Wolfe and Perren, 2001; ) ( Battarbee et al., 2002; ) Sorvari et al., 2002 [JoC] ;( Laing and Smol, 2003; ) ( Michelutti et al., 2003; ) ( Perren et al., 2003; ) ( Ruhland et al., 2003; ) ( Karst-Riddoch et al., 2005; ) Smol et al., 2005 [PoC, JoC] ). These sedimentary records also indicated changes in zooplankton communities ( Douglas et al., 1994 [JoC] ;( Battarbee et al., 2002; ) ( Korhola et al., 2002; ) ( Brooks and Birks, 2004; ) Smol et al., 2005 [PoC, JoC] ). In relatively productive lakes, there was a shift towards more diverse periphytic diatom communities due to increased macrophyte growth ( (Karst-Riddoch et al., 2005 ) ). In lakes where nutrients are becoming limited due to increased stratification, phytoplankton composition shifted to relatively fewer diatoms, potentially reducing food quality for upper trophic levels ( (Adrian and Deneke, 1996; ) Verburg et al., 2003 [JoC] ; O’Reilly, 2007 [NPR, SRC, 2007] ). Warming has also produced northward shifts in the distribution of aquatic insects and fish in the UK ( Hickling et al., 2006 [JoC] ).
Phenology
With earlier ice break-up and warmer water temperatures, some species have responded to the earlier commencement of the growing season, often advancing development of spring algal blooms as well as clear-water phases. The spring algal bloom now occurs about 4 weeks earlier in several large lakes ( (Gerten and Adrian, 2000; ) Straile and Adrian, 2000 [JoC] ;( Weyhenmeyer, 2001; ) Winder and Schindler, 2004b [JoC] ). In many cases where the spring phytoplankton bloom has advanced, zooplankton have not responded similarly, and their populations are declining because their emergence no longer corresponds with high algal abundance ( (Gerten and Adrian, 2000; ) ( Winder and Schindler, 2004a ) ). Zooplankton phenology has also been affected by climate ( (Gerten and Adrian, 2002; ) ( Winder and Schindler, 2004a ) ) and phenological shifts have also been demonstrated for some wild and farmed fish species ( (Ahas, 1999; ) ( Elliott et al., 2000 ) ). Because not all organisms respond similarly, differences in the magnitude of phenological responses among species has affected food-web interactions ( (Winder and Schindler, 2004a ) ).
Table 1.6. Examples of changes in freshwater ecosystems due to climate warming.
| Environmental factor | Observed changes | Time period considered | Location of lakes/rivers | Total number of lakes/rivers studied | Selected references |
|---|---|---|---|---|---|
| Productivity or biomass | Increases associated with longer growing season | 100 years | North America, Europe, Eastern Europe | 26 | Schindler et al., 1990; Adrian and Deneke, 1996; Gajewski et al., 1997; Weyhenmeyer et al., 1999; Straile and Adrian, 2000; Wolfe and Perren, 2001; Battarbee et al., 2002; Gerten and Adrian, 2002; Korhola et al., 2002; Carvalho and Kirika, 2003; Shimaraev and Domysheva, 2004; Winder and Schindler, 2004b; Hampton, 2005; Karst-Riddoch et al., 2005; Schindler et al., 2005 |
| Decreases due to decreased nutrient availability | 100 years | Europe, East Africa | 5 | Adrian et al., 1995; O’Reilly et al., 2003; Verburg et al., 2003; O’Reilly, 2007 | |
| Algal community composition | Shift from benthic to planktonic species | 100 to 150 years | North America, Europe | 66 | Gajewski et al., 1997; Wolfe and Perren, 2001; Battarbee et al., 2002; Sorvari et al., 2002; Laing and Smol, 2003; Michelutti et al., 2003; Perren et al., 2003; Ruhland et al., 2003; Karst-Riddoch et al., 2005; Smol et al., 2005 |
| Decreased diatom abundance | 100 years | East Africa, Europe | 3 | Adrian and Deneke, 1996; Verburg et al., 2003; O’Reilly, 2007 | |
| Phenology | Spring algal bloom up to 4 weeks earlier, earlier clear water phase | 45 years | North America, Europe | 5 | Weyhenmeyer et al., 1999; Gerten and Adrian, 2000; Straile and Adrian, 2000; Gerten and Adrian, 2002; Winder and Schindler, 2004a, 2004b |
| Fish migration | From 6 days to 6 weeks earlier | 20 to 50 years | North America | 5 | Quinn and Adams, 1996; Huntington et al., 2003; Cooke et al., 2004; Juanes et al., 2004; Lawson et al., 2004 |
1.3.4.5 Changes in rivers
In rivers, water flow can influence water chemistry, habitat, population dynamics, and water temperature ( Schindler et al., 2007 [NPR, 2007] ). Specific information on the effect of climate change on hydrology can be found in Section 1.3.2 . Increasing river temperatures have been associated with increased biological demand and decreased dissolved oxygen, without changes in flow ( Ozaki et al., 2003 [ARC] ). Riverine dissolved organic carbon concentrations have doubled in some cases because of increased carbon release in the catchment as temperature has risen ( (Worrall et al., 2003 ) ).
Abundance, distribution and migration
Climate-related changes in rivers have affected species abundance, distribution and migration patterns. While warmer water temperatures in many rivers have positively influenced the breeding success of fish ( (Fruget et al., 2001; ) ( Grenouillet et al., 2001; ) Daufresne et al., 2004 [JoC] ), the stressful period associated with higher water temperatures for salmonids has lengthened as water temperatures have increased commensurate with air temperatures in some locations ( (Bartholow, 2005 ) ). In the Rhône River there have been significant changes in species composition as southern, thermophilic fish and invertebrate species have progressively replaced cold-water species ( (Doledec et al., 1996; ) Daufresne et al., 2004 [JoC] ). Correlated with long-term increases in water temperature, the timing of fish migrations in large rivers in North America has advanced by up to 6 weeks in some years ( (Quinn and Adams, 1996; ) Huntington et al., 2003 [JoC] ;( Cooke et al., 2004; ) ( Juanes et al., 2004 ) ). Increasing air temperatures have been negatively correlated with smolt production ( (Lawson et al., 2004 ) ), and earlier migrations are associated with greater en-route and pre-spawning mortality (up to 90%) ( (Cooke et al., 2004 ) ). Warming in Alpine rivers caused altitudinal habitat shifts upward for brown trout, and there were increased incidences of temperature-dependent kidney disease at the lower-elevational habitat boundary ( Hari et al., 2006 [JoC] ).
1.3.4.6 Summary of marine and freshwater biological systems
In marine and freshwater ecosystems, many observed changes in phenology and distribution have been associated with rising water temperatures, as well as changes in salinity, oxygen levels and circulation. While there is increasing evidence for climate change impacts on coral reefs, separating the impacts of climate-related stresses from other stresses (e.g., over-fishing and pollution) is difficult. Globally, freshwater ecosystems are showing changes in organism abundance and productivity, range expansions, and phenological shifts (including earlier fish migrations) that are linked to rising temperatures. Many of these climate-related impacts are now influencing the ways in which marine and freshwater ecosystems function.
1.3.5 Terrestrial biological systems
Plants and animals can reproduce, grow and survive only within specific ranges of climatic and environmental conditions. If conditions change beyond the tolerances of species, then they may respond by:
1. shifting the timing of life-cycle events (e.g., blooming, migrating),
2. shifting range boundaries (e.g., moving poleward) or the density of individuals within their ranges,
3. changing morphology (e.g., body or egg size), reproduction or genetics,
4. extirpation or extinction.
Additionally, each species has its unique requirements for climatic and environmental conditions. Changes, therefore, can lead to disruption of biotic interaction (e.g., predator/prey) and to changes of species composition as well as ecosystem functioning. Since the TAR, the number of studies finding plants or animals responding to changing climate (associated with varying levels of confidence) has risen substantially, as has the number of reviews ( Hughes, 2000 [ARC] ; Menzel and Estrella, 2001 [NPR, SRC] ; Sparks and Menzel, 2002 [JoC, SRC] ; Walther et al., 2002 [JoC, SRC] ; Parmesan and Galbraith, 2004 [NPR] ;( Linderholm, 2006; ) ( Parmesan, 2006 ) ).
Besides climate affecting species, there are many different types of non-climate driving forces, such as invasive species, natural disturbances (e.g., wildfires), pests, diseases and pollution (e.g., soluble-nitrogen deposition), influencing the changes exhibited by species. Many animal and plant populations have been under pressure from agricultural intensification and land-use change in the past 50 years, causing many species to be in decline. Habitat fragmentation ( (Hill et al., 1999b; ) Warren et al., 2001 [JoC] ) or simply the absence of suitable areas for colonisation, e.g., at higher elevations, also play an important role ( (Wilson et al., 2005 ) ), especially in species extinction ( (Williams et al., 2003; ) Pounds et al., 2006 [JoC] ).
1.3.5.1 Changes in phenology
Phenology – the timing of seasonal activities of animals and plants – is perhaps the simplest process in which to track changes in the ecology of species in response to climate change. Observed phenological events include leaf unfolding, flowering, fruit ripening, leaf colouring, leaf fall of plants, bird migration, chorusing of amphibians, and appearance/emergence of butterflies. Numerous new studies since the TAR (reviewed by Menzel and Estrella, 2001 [NPR, SRC] ; Sparks and Menzel, 2002 [JoC, SRC] ; Walther et al., 2002 [JoC, SRC] ; Menzel, 2003 [JoC, SRC] ;( Walther, 2004 ) ) and three meta-analyses ( Parmesan and Yohe, 2003 [JoC, ARC] ; Root et al., 2003 [PoC, JoC, SRC] ; Lehikoinen et al., 2004 [SRC] ) (see Section 1.4.1 ) concurrently document a progressively earlier spring by about 2.3 to 5.2 days/decade in the last 30 years in response to recent climate warming.
Although phenological network studies differ with regard to regions, species, events observed and applied methods, their data show a clear temperature-driven extension of the growing season by up to 2 weeks in the second half of the 20th century in mid- and high northern latitudes (see Table 1.7 ), mainly due to an earlier spring, but partly due also to a later autumn. Remotely-sensed vegetation indices ( Myneni et al., 1997 [JoC] ;( Zhou et al., 2001; ) Lucht et al., 2002 [JoC, ARC] ) and analysis of the atmospheric CO2 signal ( Keeling et al., 1996 [JoC] ) confirm these findings. A corresponding longer frost-free and climatological growing season is also observed in North America and Europe (see Section 1.3.6.1 ). This lengthening of the growing season might also account for observed increases in productivity (see Section 1.3.6.2 ). The signal in autumn is less pronounced and more homogenous. The very few examples of single-station data indicate a much greater lengthening or even a shortening of the growing season ( Kozlov and Berlina, 2002 [JoC] ; Peñuelas et al., 2002 [JoC] ).
Table 1.7. Changes in length of growing season, based on observations within networks.
| Location | Period | Species/Indicator | Lengthening (days/decade) | References |
|---|---|---|---|---|
| Germany | 1951-2000 | 4 deciduous trees (LU/LC) | 1.1 to 2.3 | Menzel et al., 2001; Menzel, 2003 |
| Switzerland | 1951-1998 | 9 spring, 6 autumn phases | 2.7* | Defila and Clot, 2001 |
| Europe (Int. Phenological Gardens) | 1959-1996 1969-1998 | Various spring/autumn phases (LU to LC, LF) | 3.5 | Menzel and Fabian, 1999; Menzel, 2000; Chmielewski and Rotzer, 2001 |
| Japan | 1953-2000 | Gingko biloba (LU/LF) | 2.6 | Matsumoto et al., 2003 |
| Northern Hemisphere | 1981-1999 | Growing season by normalised difference vegetation index (NDVI) | 0.7 to 1 | Zhou et al., 2001 |
Altered timing of spring events has been reported for a broad multitude of species and locations; however, they are primarily from North America, Eurasia and Australia. Network studies where results from all sites/several species are reported, irrespective of their significance ( Table 1.8 ), show that leaf unfolding and flowering in spring and summer have, on average, advanced by 1-3 days per decade in Europe, North America and Japan over the last 30 to 50 years. Earlier flowering implies an earlier start of the pollen season (see Section 1.3.7.4 ). There are also indications that the onset of fruit ripening in early autumn has advanced in many cases ( (Jones and Davis, 2000; ) Peñuelas et al., 2002 [JoC] ; Menzel, 2003 [JoC, SRC] ) (see also Section 1.3.6.1 ). Spring and summer phenology is sensitive to climate and local weather ( Sparks et al., 2000 [SRC] ; Lucht et al., 2002 [JoC, ARC] ; Menzel, 2003 [JoC, SRC] ). In contrast to autumn phenology ( Estrella and Menzel, 2006 [SRC] ), their climate signal is fairly well understood: nearly all spring and summer changes in plants, including agricultural crops ( Estrella et al., 2007 [NPR, SRC, 2007] ), correlate with spring temperatures in the preceding months. The advancement is estimated as 1 to12 days for every 1°C increase in spring temperature, with average values ranging between 2.5 and 6 days per °C (e.g., ( Chmielewski and Rotzer, 2001; ) Menzel, 2003 [JoC, SRC] ; Donnelly et al., 2004 [ARC] ; Menzel et al., 2006b [JoC, SRC] ). Alpine species are also partly sensitive to photoperiod ( Keller and Korner, 2003 [ARC] ) or amount of snowpack ( (Inouye et al., 2002 ) ). Earlier spring events and a longer growing season in Europe are most apparent for time-series ending in the mid- 1980 s or later ( Schaber, 2002 [NPR, MoS] ; Scheifinger et al., 2002 [JoC, SRC] ; Dose and Menzel, 2004 [JoC, SRC] ; Menzel and Dose, 2005 [MoS, SRC] ), which matches the turning points in the respective spring temperature series ( Dose and Menzel, 2006 [JoC, SRC] ).
Table 1.8. Changes in the timing of spring events, based on observations within networks.
| Location | Period | Species/Indicator | Observed changes (days/decade) | References |
|---|---|---|---|---|
| Western USA | 1957-1994 | Lilac, honeysuckle (F) | -1.5 (lilac), 3.5 (honeysuckle) | Cayan et al., 2001 |
| North-eastern USA | 1965-2001 1959-1993 | Lilac (F, LU) Lilac (F) | -3.4 (F) -2.6 (U) -1.7 | Wolfe et al., 2005 Schwartz and Reiter, 2000 |
| Washington, DC | 1970-1999 | 100 plant species (F) | -0.8 | Abu-Asab et al., 2001 |
| Germany | 1951-2000 | 10 spring phases (F, LU) | -1.6 | Menzel et al., 2003 |
| Switzerland | 1951-1998 | 9 spring phases (F, LU) | -2.3 (*) | Defila and Clot, 2001 |
| South-central England | 1954-2000 | 385 species (F) | -4.5 days in 1990s | Fitter and Fitter, 2002 |
| Europe (Int. Phenological Gardens) | 1959-1996 1969-1998 | Different spring phases (F, LU) | -2.1 -2.7 | Menzel and Fabian, 1999; Menzel, 2000; Chmielewski and Rotzer, 2001 |
| 21 European countries | 1971-2000 | F, LU of various plants | -2.5 | Menzel et al., 2006b |
| Japan | 1953-2000 | Gingko biloba (LU) | -0.9 | Matsumoto et al., 2003 |
| Northern Eurasia | 1982-2004 | NDVI | -1.5 | Delbart et al., 2006 |
| UK | 1976-1998 | Butterfly appearance | -2.8 to -3.2 | Roy and Sparks, 2000 |
| Europe, N. America | Past 30-60 years | Spring migration of bird species | -1.3 to -4.4 | Crick et al., 1997; Crick and Sparks, 1999; Dunn and Winkler, 1999; Inouye et al., 2000; Bairlein and Winkel, 2001; Lehikoinen et al., 2004 |
| N. America (US-MA) | 1932-1993 | Spring arrival, 52 bird species | +0.8 to -9.6 (*) | Butler, 2003 |
| N. America (US-IL) | 1976-2002 | Arrival, 8 warbler species | +2.4 to -8.6 | Strode, 2003 |
| England (Oxfordshire) | 1971-2000 | Long-distance migration, 20 species | +0.4 to -6.7 | Cotton, 2003 |
| N. America (US-MA) | 1970-2002 | Spring arrival,16 bird species | -2.6 to -10.0 | Ledneva et al., 2004 |
| Sweden (Ottenby) | 1971-2002 | Spring arrival, 36 bird species | +2.1 to -3.0 | Stervander et al., 2005 |
| Europe | 1980-2002 | Egg-laying, 1 species | -1.7 to -4.6 | Both et al., 2004 |
| Australia | 1970-1999 | 11 migratory birds | 9 species earlier arrival | Green and Pickering, 2002 |
| Australia | 1984-2003 | 2 spring migratory birds | 1 species earlier arrival | Chambers et al., 2005 |
Records of the return dates of migrant birds have shown changes in recent decades associated with changes in temperature in wintering or breeding grounds or on the migration route ( Tryjanowski, 2002 [SRC] ;( Butler, 2003; ) Cotton, 2003 [JoC] ;( Huppop and Huppop, 2003 ) ). For example, a 2 to 3 day earlier arrival with a 1°C increase in March temperature is estimated for the swallow in the UK ( Sparks and Loxton, 1999 [NPR, SRC] ) and Ireland ( Donnelly et al., 2004 [ARC] ). Different measurement methods, such as first observed individual, beginning of sustained migratory period, or median of the migratory period, provide different information about the natural history of different species ( (Sokolov et al., 1998; ) Sparks and Braslavska, 2001 [SRC] ;( Huppop and Huppop, 2003; ) Tryjanowski et al., 2005 [SRC] ).
Egg-laying dates have advanced in many bird species ( (Hussell, 2003; ) ( Dunn, 2004 ) ). The confidence in such studies is enhanced when the data cover periods/sites of both local cooling and warming. Flycatchers in Europe ( (Both et al., 2004 ) ) provide such an example, where the trend in egg-laying dates matches trends in local temperatures. Many small mammals have been found to come out of hibernation and to breed earlier in the spring now than they did a few decades ago ( Inouye et al., 2000 [JoC] ;( Franken and Hik, 2004 ) ). Larger mammals, such as reindeer, are also showing phenological changes ( Post and Forchhammer, 2002 [JoC] ), as are butterflies, crickets, aphids and hoverflies ( Forister and Shapiro, 2003 [JoC] ; Stefanescu et al., 2003 [JoC] ; Hickling et al., 2005 [JoC] ; Newman, 2005 [JoC] ). Increasing regional temperatures are also associated with earlier calling and mating and shorter time to maturity of amphibians ( (Gibbs and Breisch, 2001; ) Reading, 2003 [NPR] ; Tryjanowski et al., 2003 [SRC] ). Despite the bulk of evidence in support of earlier breeding activity as a response to temperature, counter-examples also exist ( Blaustein et al., 2001 [SRC] ).
Changes in spring and summer activities vary by species and by time of season. Early-season plant species exhibit the strongest reactions ( (Abu-Asab et al., 2001; ) Menzel et al., 2001 [Ambiguous] ; Fitter and Fitter, 2002 [JoC] ; Sparks and Menzel, 2002 [JoC, SRC] ; Menzel, 2003 [JoC, SRC] ). Short-distance migrating birds often exhibit a trend towards earlier arrival, while the response of later-arriving long-distance migrants is more complex, with many species showing no change, or even delayed arrival ( (Butler, 2003; ) Strode, 2003 [JoC] ). Annual plants respond more strongly than congeneric perennials, insect-pollinated more than wind-pollinated plants, and woody less than herbaceous plants ( Fitter and Fitter, 2002 [JoC] ). Small-scale spatial variability may be due to microclimate, land cover, genetic differentiation, and other non-climate drivers ( Menzel et al., 2001 [Ambiguous] ; Menzel, 2002 [JoC, SRC] ). Large-scale geographical variations in the observed changes are found in China with latitude ( Chen et al., 2005a [JoC] ), in Switzerland with altitude ( Defila and Clot, 2001 [ARC] ) and in Europe with magnitude of temperature change ( Menzel and Fabian, 1999 [JoC, SRC] ; Sparks et al., 1999 [Ambiguous] ). Spring advance, being more pronounced in maritime western and central Europe than in the continental east ( Ahas et al., 2002 [JoC, SRC] ), is associated with higher spatial variability ( Menzel et al., 2006a [SRC] ).
As the North Atlantic Oscillation (NAO) is correlated with temperature (see Trenberth et al., 2007 [NPR, PoC, 2007] ), the NAO has widespread influence on many ecological processes. For example, the speed and pattern ( Menzel et al., 2005b [JoC, SRC] ), as well as recent trends of spring events in European plants, has also changed consistently with changes seen in the NAO index ( (Chmielewski and Rotzer, 2001; ) Scheifinger et al., 2002 [JoC, SRC] ; Walther et al., 2002 [JoC, SRC] ; Menzel, 2003 [JoC, SRC] ). Similarly, earlier arrival and breeding of migratory birds in Europe are often related to warmer local temperatures and higher NAO indices ( (Hubalek, 2003; ) ( Huppop and Huppop, 2003; ) ( Sanz, 2003 ) ). However, the directions of changes in birds corresponding to NAO can differ across Europe ( (Hubalek, 2003; ) Kanuscak et al., 2004 [SRC] ). Likewise, the relevance of the NAO index on the phenology of plants differs across Europe, being more pronounced in the western (France, Ireland, UK) and north-western (south Scandinavia) parts of Europe and less distinct in the continental part of Europe (see Figure 1.4 a; Menzel et al., 2005b [JoC, SRC] ). In conclusion, spring phenological changes in birds and plants and their triggering by spring temperature are often similar, as described in some cross-system studies; however, the NAO influence is weaker than the temperature trigger and is restricted to certain time periods ( Walther et al., 2002 [JoC, SRC] ) ( Figure 1.4 b).
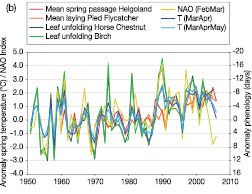
Figure 1.4. (a) Differences between the mean onset of spring (days) in Europe for the 10 years with the highest ( 1990, 1882, 1928, 1903, 1993, 1910, 1880, 1997, 1989, 1992 ) and the lowest ( 1969, 1936, 1900, 1996, 1960, 1932, 1886, 1924, 1941, 1895 ) NAO winter and spring index (November to March) drawn from the period 1879 to 1998 . After Menzel et al. 2005b [JoC, SRC] ). (b) Anomalies of different phenological phases in Germany (mean spring passage of birds at Helgoland, North Sea; mean egg-laying of pied flycatcher in Northern Germany; national mean onset of leaf unfolding of common horse-chestnut (Aesculus hippocastanum) and silver birch (Betula pendula) (negative = earlier)), anomalies of mean spring air temperature T (HadCRUT3v) and North Atlantic Oscillation index (NAO) (http://www.cru.uea.ac.uk/cru/data/). Updated after Walther et al. 2002 [JoC, SRC] ).
1.3.5.2 Changes in species distributions and abundances
Many studies of species abundances and distributions corroborate predicted systematic shifts related to changes in climatic regimes, often via species-specific physiological thresholds of temperature and precipitation tolerance. Habitat loss and fragmentation may also influence these shifts. Empirical evidence shows that the natural reaction of species to climate change is hampered by habitat fragmentation and/or loss ( (Hill et al., 1999b; ) Warren et al., 2001 [JoC] ;( Opdam and Wascher, 2004 ) ). However, temperature is likely to be the main driver if different species in many different areas, or species throughout broad regions, shift in a co-ordinated and systematic manner. In particular, some butterflies appear to track decadal warming quickly ( Parmesan et al., 1999 [JoC] ), whereas the sensitivity of tree-line forests to climate warming varies with topography and the tree-line history (e.g., human impacts) ( (Holtmeier and Broll, 2005 ) ). Several different bird species no longer migrate out of Europe in the winter as the temperature continues to warm. Additionally, many species have recently expanded their ranges polewards as these higher-latitude habitats become less marginal ( Thomas et al., 2001a [JoC] ). Various studies also found connections between local ecological observations across diverse taxa (birds, mammals, fish) and large-scale climate variations associated with the North Atlantic Oscillation (NAO), El Niño-Southern Oscillation (ENSO), and Pacific Decadal Oscillation ( Blenckner and Hillebrand, 2002 [JoC] ). For example, the NAO and/or ENSO has been associated with the synchronisation of population dynamics of caribou and musk oxen ( Post and Forchhammer, 2002 [JoC] ), reindeer calf survival ( (Weladji and Holand, 2003 ) ), fish abundance ( (Guisande et al., 2004 ) ), fish range shifts ( (Dul?i? et al., 2004 ) ) and avian demographic dynamics ( (Sydeman et al., 2001; ) ( Jones et al., 2002; ) ( Almaraz and Amat, 2004 ) ).
Changes in the distribution of species have occurred across a wide range of taxonomic groups and geographical locations during the 20th century ( Table 1.9 ). Over the past decades, a poleward extension of various species has been observed, which is probably attributable to increases in temperature ( Parmesan and Yohe, 2003 [JoC, ARC] ). One cause of these expansions is increased survivorship ( (Crozier, 2004 ) ). Many Arctic and tundra communities are affected and have been replaced by trees and dwarf shrubs ( (Kullman, 2002; ) ACIA, 2005 [NPR] ). In north-western Europe, e.g., in the Netherlands ( Tamis et al., 2001 [NPR] ) and central Norway ( EEA, 2004 [NPR] ), thermophilic (warmth-requiring) plant species have become significantly more frequent compared with 30 years ago. In contrast, there has been a small decline in the presence of traditionally cold-tolerant species. These changes in composition are the result of the migration of thermophilic species into these new areas, but are also due to an increased abundance of these species in their current locations.
Table 1.9. Evidence of significant recent range shifts polewards and to higher elevations.
| Location | Species/Indicator | Observed range shift due to increased temperature (if nothing else stated) | References |
|---|---|---|---|
| California coast, USA | Spittlebug | Northward range shift | Karban and Strauss, 2004 |
| Sweden Czech Republic | Tick (Ixodes ricinus) | Northward expansion 1982-1996 Expansion to higher altitudes (+300 m) | Lindgren et al., 2000 Daniel et al., 2003 |
| Washington State, USA | Skipper butterfly | Range expansion with increased Tmin | Crozier, 2004 |
| UK | 329 species across 16 taxa | Northwards (av. 31-60 km) and upwards (+25 m) in 25 years. Significant northwards and elevational shifts in 12 of 16 taxa. Only 3 species of amphibians and reptiles shifted significantly southwards and to lower elevations | Hickling et al., 2006 |
| UK | Speckled wood (Pararge aegeria) | Expanded northern margin, at 0.51-0.93 km/yr, depending on habitat availability | Hill et al., 2001 |
| UK | 4 northern butterflies (1970-2004) | 2 species retreating 73 and 80 km north, 1 species retreating 149 m uphill | Franco et al., 2006 |
| Central Spain | 16 butterfly species | Upward shift of 210 m in the lower elevational limit between 1967-73 and 2004 | Wilson et al., 2005 |
| Britain | 37 dragonfly and damselfly species | 36 out of 37 species shifted northwards (mean 84 km) from 1960-70 to 1985-95 | Hickling et al., 2005 |
| Czech Republic | 15 of 120 butterfly species | Uphill shifts in last 40 years | Konvicka et al., 2003 |
| Poland | White stork (Ciconia ciconia) | Range expansions in elevation, 240 m during last 70 years | Tryjanowski et al., 2005 |
| Australia | 3 macropods and 4 feral mammal species | Range expansions to higher altitudes | Green and Pickering, 2002 |
| Australia | Grey-headed flying fox | Contraction of southern boundary poleward by 750 km since 1930s | Tidemann et al., 1999 |
| Senegal, West Africa | 126 tree and shrub species (1945-1993) | Up to 600 m/yr latitudinal shift of ecological zones due to decrease in precipitation | Gonzalez, 2001 |
| Russia, Bulgaria, Sweden, Spain, New Zealand, USA | Tree line | Advancement towards higher altitudes | Meshinev et al., 2000; Kullman, 2002; Peñuelas and Boada, 2003; Millar and Herdman, 2004 |
| Canada | Bioclimatic taiga-tundra ecotone indicator | 12 km/yr northward shift (NDVI data) | Fillol and Royer, 2003 |
| Alaska | Arctic shrub vegetation | Expansion into previously shrub-free areas | Sturm et al., 2001 |
| European Alps | Alpine summit vegetation | Elevational shift, increased species-richness on mountain tops | Grabherr et al., 2001; Pauli et al., 2001; Walther et al., 2005a |
| Montana, USA | Arctic-alpine species | Decline at the southern margin of range | Lesica and McCune, 2004 |
| Germany, Scandinavia | English holly (Ilex aquifolium) | Poleward shift of northern margin due to increasing winter temperatures | Walther et al., 2005b |
Altitudinal shifts of plant species have been well documented ( Grabherr et al., 2001 [NPR] ;( Dobbertin et al., 2005; ) ( Walther et al., 2005a ) ) ( Table 1.9 ). In several Northern Hemisphere mountain systems, tree lines have markedly shifted to higher elevations during the 20th century, such as in the Urals (Moiseev and Shiyatov, 2003 [NotFound] ), in Bulgaria ( (Meshinev et al., 2000 ) ), in the Scandes Mountains of Scandinavia ( (Kullman, 2002 ) ) and in Alaska ( Sturm et al., 2001 [JoC] ). In some places, the position of the tree line has not extended upwards in elevation in the last half-century ( (Cullen et al., 2001; ) ( Masek, 2001; ) ( Klasner and Fagre, 2002 ) ), which may be due to time-lag effects owing to poor seed production/dispersal, to the presence of ‘surrogate habitats’ with special microclimates, or to topographical factors ( (Holtmeier and Broll, 2005 ) ). In mountainous regions, climate is a main driver of species composition, but in some areas, grazing, logging or firewood collection can be of considerable relevance. In parts of the European Alps, for example, the tree line is influenced by past and present land-use impacts ( Theurillat and Guisan, 2001 [JoC, ARC] ;( Carnelli et al., 2004 ) ). A climate warming-induced upward migration of alpine plants in the high Alps ( Grabherr et al., 2001 [NPR] ; Pauli et al., 2001 [NPR] ) was observed to have accelerated towards the beginning of the 21st century ( (Walther et al., 2005a ) ). Species ranges of alpine plants also have extended to higher altitudes in the Norwegian Scandes ( (Klanderud and Birks, 2003 ) ). Species in alpine regions, which are often endemic and of high importance for plant diversity ( Vare et al., 2003 [NPR] ), are vulnerable to climate warming, most probably because of often restricted climatic ranges, small isolated populations, and the absence of suitable areas at higher elevations in which to migrate ( Pauli et al., 2003 [NPR] ).
1.3.5.3 Climate-linked extinctions and invasions
Key indicators of a species’ risk of extinction (global loss of all individuals) or extirpation (loss of a population in a given location) include the size of its range, the density of individuals within the range, and the abundance of its preferred habitat within its range. Decreases in any of these factors (e.g., declining range size with habitat fragmentation) can lower species population size ( Wilson et al., 2004 [JoC] ). Each of these factors can be directly affected by rapid global warming, but the causes of extinctions/extirpations are most often multifactorial. For example, a recent extinction of around 75 species of frogs, endemic to the American tropics, was most probably due to a pathogenic fungus (Batrachochytrium), outbreaks of which have been greatly enhanced by global warming ( Pounds et al., 2006 [JoC] ). Other examples of declines in populations and subsequent extinction/extirpation are found in amphibians around the world ( (Alexander and Eischeid, 2001; ) ( Middleton et al., 2001; ) ( Ron et al., 2003; ) ( Burrowes et al., 2004 ) ). Increasing climatic variability, linked to climate change, has been found to have a significant impact on the extinction of the butterfly Euphydryas editha bayensis ( McLaughlin et al., 2002a [JoC] , 2002b ). Currently about 20% of bird species (about 1,800) are threatened with extinction, while around 5% are already functionally extinct (e.g., small inbred populations) ( Sekercioglu et al., 2004 [JoC] ). The pika (Ochotona princeps), a small mammal found in mountains of the western USA, has been extirpated from many slopes ( (Beever et al., 2003 ) ). New evidence suggests that climate-driven extinctions and range retractions are already widespread, which have been poorly reported due, at least partly, to a failure to survey the distributions of species at sufficiently fine resolution to detect declines and to attribute such declines to climate change ( (Thomas et al., 2006 ) ).
A prominent cause of range contraction or loss of preferred habitat within a species range is invasion by non-native species. Fluctuation in resource availability, which can be driven by climate, has been identified as the key factor controlling invasibility ( (Davis et al., 2000 ) ). The clearest evidence for climate variability triggering an invasion occurs where a suite of species with different histories of introduction spread en-masse during periods of climatic amelioration ( Walther, 2000 [MoS] ; Walther et al., 2002 [JoC, SRC] ). Climate change will greatly affect indigenous species on sub-Antarctic islands, primarily due to warmer climates allowing exotic species, such as the house mouse (Mus musculus) and springtails (Collembola spp.), to become established and proliferate ( Smith, 2002 [JoC] ). A prominent example is that of exotic thermophilous plants spreading into the native flora of Spain, Ireland and Switzerland ( Pilcher and Hall, 2001 [NPR] ; Sobrino et al., 2001 [NPR] ). Elevated CO2 might also contribute to the spread of weedy, non-indigenous plants ( Hattenschwiler and Korner, 2003 [ARC] ).
1.3.5.4 Changes in morphology and reproduction
A change in fecundity is one of the mechanisms altering species distributions (see Section 1.3.5.2 ). Temperature can affect butterfly egg-laying rate and microhabitat selection; recent warming has been shown to increase egg-laying and thus population size for one species ( (Davies et al., 2006 ) ). The egg sizes of many bird species are changing with increasing regional temperatures, but the direction of change varies by species and location. For example, in Europe, the egg size of pied flycatchers increased with regional warming ( (Jarvinen, 1994, ) 1996 ). In southern Poland, the size of red-backed shrikes’ eggs has decreased, probably due to decreasing female body size, which is also associated with increasing temperatures ( Tryjanowski et al., 2004 [SRC] ). The eggs of European barn swallows are getting larger with increasing temperatures and their breeding season is occurring earlier. Additionally, in the eggs, concentrations of certain maternally supplied nutrients, such as those affecting hatchability, viability and parasite defence, have also increased with warming ( Saino et al., 2004 [NPR] ). Studies from eastern Poland, Asia, Europe and Japan have found that various birds and mammals exhibit trends toward larger body size, probably due to increasing food availability, with regionally increasing temperatures ( (Nowakowski, 2002; ) ( Yom-Tov, 2003; ) Kanuscak et al., 2004 [SRC] ;( Yom-Tov and Yom-Tov, 2004 ) ). Reproductive success in polar bears has declined, resulting in a drop in body condition, which in turn is due to melting Arctic Sea ice. Without ice, polar bears cannot hunt seals, their favourite prey ( Derocher et al., 2004 [ARC] ).
These types of changes are also found in insects and plants. The evolutionary lengthening and strengthening of the wings of some European Orthoptera and butterflies has facilitated their northward range expansion but has decreased reproductive output ( (Hill et al., 1999a; ) Thomas et al., 2001a [JoC] ;( Hughes et al., 2003a; ) ( Simmons and Thomas, 2004 ) ). The timing and duration of the pollen season, as well as the amount of pollen produced ( Beggs, 2004 [MoS, ARC] ), have been found to be affected by regional warming (see Section 1.3.7.4 ).
1.3.5.5 Species community changes and ecosystem processes
In many parts of the world, species composition has changed ( Walther et al., 2002 [JoC, SRC] ), partly due to invasions and distributional changes. The assemblages of species in ecological communities reflect interactions among organisms as well as between organisms and the abiotic environment. Climate change, extreme climatic events or other processes can alter the composition of species in an ecosystem because species differentially track their climate tolerances. As species in a natural community do not respond in synchrony to such external pressures, ecological communities existing today could easily be disaggregated ( Root and Schneider, 2002 [NPR, PoC, SRC] ).
Species diversity in various regions is changing due to the number of species shifting, invading or receding ( Tamis et al., 2001 [NPR] ; EEA, 2004 [NPR] ) (see Sections 1.3.5.2 and 1.3.5.3 ). Average species richness of butterflies per 20 km grid cell in the UK increased between 1970 - 1982 and 1995 - 1999, but less rapidly than would have been expected had all species been able to keep up with climate change ( (Menendez et al., 2006 ) ). In non-fragmented Amazon forests, direct effects of CO2 on photosynthesis, as well as faster forest turnover rates, may have caused a substantial increase in the density of lianas over the last two decades ( Phillips et al., 2004 [JoC] ). Although many species-community changes are also attributable to landscape fragmentation, habitat modification and other non-climate drivers, many studies show a high correlation between changes in species composition and recent climate change, also via the frequency of weather-based disturbances ( Hughes, 2000 [ARC] ; Pauli et al., 2001 [NPR] ; Parmesan and Yohe, 2003 [JoC, ARC] ). Examples of altered or stable synchrony in ecosystems via multi-species interactions, e.g., the pedunculate oak–winter moth–tit food chain, are still fairly rare ( (van Noordwijk et al., 1995; ) ( Buse et al., 1999 ) ).
1.3.5.6 Species evolutionary processes
Recent evolutionary responses to climate change have been addressed in reviews ( Thomas, 2005 [NPR] ; Bradshaw and Holzapfel, 2006 [JoC] ). Changes have taken place in the plants preferred for egg-laying and feeding of butterflies, e.g., a broadened diet facilitated the colonisation of new habitats during range extension in the UK ( Thomas et al., 2001a [JoC] ). The pitcher-plant mosquito in the USA has prolonged development time in late summer by the evolution of changed responses to day length ( Bradshaw and Holzapfel, 2001 [JoC] ;( Bradshaw et al., 2003 ) ). The blackcap warbler has recently extended its overwintering range northwards in Europe by evolving a change in migration direction ( Berthold et al., 2003 [NPR] ). Insects expanding their ranges have undertaken genetically-based changes in dispersal morphology, behaviour and other life-history traits, as ‘good colonists’ have been at a selective advantage ( (Hill et al., 1999a; ) Thomas et al., 2001b [JoC] ;( Hughes et al., 2003a; ) ( Simmons and Thomas, 2004 ) ). Genetic changes in Drosophila melanogaster in eastern coastal Australia over 20 years are likely to reflect increasingly warmer and drier conditions ( Umina et al., 2005 [JoC] ). Evolutionary processes are also demonstrated in the timing of reproduction associated with climate change in North American red squirrels ( (Berteaux et al., 2004 ) ). There is no evidence so far that the temperature response rates of plants have changed over the last century ( Menzel et al., 2005a [SRC] ).
While a variety of methods have been used that provide evidence of biological change over many ecosystems, there remains a notable absence of studies on some ecosystems, particularly those in tropical regions, due to a significant lack of long-term data. Furthermore, not all processes influenced by warming have yet been studied. Nevertheless, in the large majority of studies, the observed trends found in species correspond to predicted changes in response to regional warming in terms of magnitude and direction. Analyses of regional differences in trends reveal that spatio-temporal patterns of both phenological and range changes are consistent with spatio-temporal patterns expected from observed climate change.
1.3.5.7 Summary of terrestrial biological systems
The vast majority of studies of terrestrial biological systems reveal notable impacts of global warming over the last three to five decades, which are consistent across plant and animal taxa: earlier spring and summer phenology and longer growing seasons in mid- and higher latitudes, production range expansions at higher elevations and latitudes, some evidence for population declines at lower elevational or latitudinal limits to species ranges, and vulnerability of species with restricted ranges, leading to local extinctions. Non-climate synergistic factors can significantly limit migration and acclimatisation capacities.
1.3.6 Agriculture and forestry
Although agriculture and forestry are known to be highly dependent on climate, little evidence of observed changes related to regional climate changes was noted in the TAR. This is probably due to the strong influence of non-climate factors on agriculture and, to a lesser extent, on forestry, especially management practices and technological changes, as well as market prices and policies related to subsidies ( Easterling, 2003 [NPR, ARC] ). The worldwide trends in increasing productivity (yield per hectare) of most crops over the last 40 years, primarily due to technological improvements in breeding, pest and disease control, fertilisation and mechanisation, also make identifying climate-change signals difficult ( (Hafner, 2003 ) ).
1.3.6.1 Crops and livestock
Changes in crop phenology provide important evidence of responses to recent regional climate change ( Table 1.10 ). Such changes are apparent in perennial crops, such as fruit trees and wine-making varieties of grapes, which are less dependent on yearly management decisions by farmers than annual crops and are also often easier to observe. Phenological changes are often observed in tandem with changes in management practices by farmers. A study in Germany ( Menzel et al., 2006c [SRC] ) has revealed that between 1951 and 2004 the advance for agricultural crops (2.1 days/decade) has been significantly less marked than for wild plants or fruit trees (4.4 to 7.1 days/decade). All the reported studies concern Europe, where recent warming has clearly advanced a significant part of the agricultural calendar.
Table 1.10. Observed changes in agricultural crop and livestock.
| Agricultural metric | Observed change | Location | Period | References |
|---|---|---|---|---|
| Phenology | Advance of stem elongation for winter rye (10 days) and emergence for maize (12 days) | Germany | 1961-2000 | Chmielewski et al., 2004 |
| Advance in cherry tree flowering (0.9 days/10 years), apple tree flowering (1.1 days/10 years) in response (-5 days/°C) to March/April temperature increase | 1951-2000 | Menzel, 2003 | ||
| Advance in beginning of growing season of fruit trees (2.3 days/10 years), cherry tree blossom (2.0 days/10 years), apple tree blossom (2.2 days/10 years) in agreement with 1.4°C annual air temperature increase | 1961-1990 | Chmielewski et al., 2004 | ||
| Advance of fruit tree flowering of 1-3 weeks for apricot and peach trees, increase in spring frost risks and more frequent occurrence of bud fall or necrosis for sensitive apricot varieties | South of France | 1970-2001 | Seguin et al., 2004 | |
| Management practices, pests and diseases | Advance of seeding dates for maize and sugarbeet (10 days) | Germany | 1961-2000 | Chmielewski et al., 2004 |
| Advance of maize sowing dates by 20 days at 4 INRA experimental farms | France | 1974-2003 | Benoit and Torre, 2004 | |
| Advance of potato sowing date by 5 days, no change for spring cereals | Finland | 1965-1999 | Hilden et al., 2005 | |
| Partial shift of apple codling moth from 2 to 3 generations | South of France | 1984-2003 | Sauphanor and Boivin, 2004 | |
| Yields | Lower hay yields, in relation to warmer summers | Rothamsted UK | 1965-1998 | Cannell et al., 1999 |
| Part of overall yield increase attributed to recent cooling during growing season: 25% maize, 33% soybean | USA county level | 1982-1998 | Lobell and Asner, 2003 | |
| Decrease of rice yield associated with increase in temperature (0.35°C and 1.13°C for Tmax and Tmin, respectively, during 1979 to 2003) | Philippines | 1992-2003 | Peng et al., 2004 | |
| Livestock | Decrease of measured pasture biomass by 20-30% | Mongolia | 1970-2002 | Batimaa, 2005 |
| Decline of NDVI of the third period of 10 days of July by 69% for the whole territory | 1982-2002 | Erdenetuya, 2004 | ||
| Observed increase in animal production related to warming in summer and annual temperature | Tibet | 1978-2002 | Du et al., 2004 |
Since the TAR, there has been evidence of recent trends in agro-climatic indices, particularly those with a direct relationship to temperature, such as increases in growing season length and in growing-degree-days during the crop cycle. These increases, associated with earlier last spring frost and delayed autumn frost dates, are clearly apparent in temperate regions of Eurasia ( (Moonen et al., 2002; ) Menzel et al., 2003 [JoC, SRC] ; Genovese et al., 2005 [NPR, MoS] ; Semenov et al., 2006 [NPR, ARC] ) and a major part of North America ( Robeson, 2002 [JoC] ;( Feng and Hu, 2004 ) ). They are especially detectable in indices applicable to wine-grape cultivation ( Box 1.2 ). In Sahelian countries, increasing temperature in combination with rainfall reduction has led to a reduced length of vegetative period, no longer allowing present varieties to complete their cycle ( Ben Mohamed et al., 2002 [JoC] ).
However, no detectable change in crop yield directly attributable to climate change has been reported for Europe. For example, the yield trend of winter wheat displays progressive growth from 2.0 t/ha in 1961 to 5.0 t/ha in 2000, with anomalies due to climate variability on the order of 0.2 t/ha ( (Cantelaube et al., 2004 ) ). The same observation is valid for Asia, where the rice production of India has grown over the period 1950 - 1999 from 20 Mt to over 90 Mt, with only a slight decline during El Niño years when monsoon rainfall is reduced ( Selvaraju, 2003 [JoC] ). A negative effect of warming for rice production observed by the International Rice Research Institute (IRRI) in the Philippines (yield loss of 15% for 1°C increase of growing-season minimum temperature in the dry season) ( Peng et al., 2004 [JoC] ) is limited to a local observation for a short time period; a similar effect has been noted on hay yield in the UK (1°C increase in July-August led to a 0.33 t/ha loss) ( Cannell et al., 1999 [NPR, SRC] ). A study at the county level of U.S. maize and soybean yields ( Lobell and Asner, 2003 [JoC] ) has established a positive effect of cooler and wetter years in the Midwest and hotter and drier years in the North-west plains. In the case of the Sahel region of Africa, warmer and drier conditions have served as a catalyst for a number of other factors that have accelerated a decline in groundnut production ( Van Duivenbooden et al., 2002 [JoC] ).
For livestock, one study in Tibet reports a significant relationship of improved performance with warming in high mountainous conditions ( (Du et al., 2004 ) ). On the other hand, the pasture biomass in Mongolia has been affected by the warmer and drier climate, as observed at a local station ( Batimaa, 2005 [NPR] ) or at the regional scale by remote sensing ( Erdenetuya, 2004 [NPR] ).
Box 1.2. Wine and recent warming
Wine-grapes are known to be highly sensitive to climatic conditions, especially temperature (e.g., viticulture was thriving in England during the last medieval warm period). They have been used as an indicator of observed changes in agriculture related to warming trends, particularly in Europe and in some areas of North America.
In Alsace, France, the number of days with a mean daily temperature above 10°C (favourable for vine activity) has increased from 170 around 1970 to 210 at the end of the 20th century ( (Duchêne and Schneider, 2005 ) ). An increase associated with a lower year-to-year variability in the last 15 years of the heliothermal index of Huglin ( Seguin et al., 2004 [SRC] ) has been observed for all the wine-producing areas of France, documenting favourable conditions for wine, in terms of both quality and stability. Similar trends in the average growing-season temperatures (April-October for the Northern Hemisphere) have been observed at the main sites of viticultural production in Europe ( (Jones, 2005 ) ). The same tendencies have also been found in the California, Oregon and Washington vineyards of the USA ( Nemani et al., 2001 [ARC] ;( Jones, 2005 ) ).
The consequences of warming are already detectable in wine quality, as shown by ( Duchêne and Schneider 2005 ) ), with a gradual increase in the potential alcohol levels at harvest for Riesling in Alsace of nearly 2% volume in the last 30 years. On a worldwide scale, for 25 of the 30 analysed regions, increasing trends of vintage ratings (average rise of 13.3 points on a 100-point scale for every 1°C warmer during the growing season), with lower vintage-to-vintage variation, has been established ( (Jones, 2005 ) ).
1.3.6.2 Forestry
Here we focus on forest productivity and its contributing factors (see Section 1.3.5 for phenological aspects). Rising atmospheric CO2 concentration, lengthening of the growing season due to warming, nitrogen deposition and changed management have resulted in a steady increase in annual forest CO2 storage capacity in the past few decades, which has led to a more significant net carbon uptake ( Nabuurs et al., 2002 [JoC, MoS] ). Satellite-derived estimates of global net primary production from satellite data of vegetation indexes indicate a 6% increase from 1982 to 1999, with large increases in tropical ecosystems ( Nemani et al., 2003 [JoC, ARC] ) ( Figure 1.5 ). The study by Zhou et al. 2003 [JoC] ), also using satellite data, confirm that the Northern Hemisphere vegetation activity has increased in magnitude by 12% in Eurasia and by 8% in North America from 1981 to 1999 . Thus, the overall trend towards longer growing seasons is consistent with an increase in the ‘greenness’ of vegetation, for broadly continuous regions in Eurasia and in a more fragmented way in North America, reflecting changes in biological activity. Analyses in China attribute increases in net primary productivity, in part, to a country-wide lengthening of the growing season ( (Fang and Dingbo, 2003 ) ). Similarly, other studies find a decrease of 10 days in the frost period in northern China ( (Schwartz and Chen, 2002 ) ) and advances in spring phenology ( (Zheng et al., 2002 ) ).
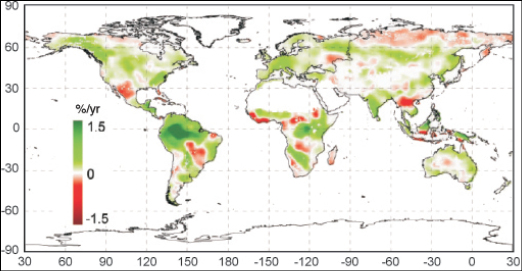
Figure 1.5. Estimated changes in net primary productivity (NPP) between 1982 and 1999 derived from independent NDVI data sets from the Global Inventory Modeling and Mapping Studies (GIMMS) and Pathfinder Advanced Very High Resolution Radiometer (AVHRR) Land (PAL). An overall increase in NPP is observed, which is consistent with rising atmospheric CO2 and warming. From Nemani et al., 2003 [JoC, ARC] . Reprinted with permission from AAAS.
However, in the humid evergreen tropical forest in Costa Rica, annual growth from 1984 to 2000 was shown to vary inversely with the annual mean of daily minimum temperature, because of increased respiration at night ( Clark et al., 2003 [JoC] ). For southern Europe, a trend towards a reduction in biomass production has been detected in relation to rainfall decrease ( (Maselli, 2004 ) ), especially after the severe drought of 2003 ( (Gobron et al., 2005; ) ( Lobo and Maisongrande, 2006 ) ). A recent study in the mountains of north-east Spain ( Jump et al., 2006 [NPR] ) shows significantly lower growth of mature beech trees at the lower limit of this species compared with those at higher altitudes. Growth at the lower Fagus limit was characterised by a rapid recent decline starting in approximately 1975 . By 2003, the growth of mature trees had fallen by 49% when compared with pre-decline levels. Analysis of climate–growth relationships suggests that the observed decline in growth is a result of warming temperatures. For North America, recent observations from satellite imagery (for the period 1982 to 2003 ) document a decline for a substantial portion of northern forest, possibly related to warmer and longer summers, whereas tundra productivity is continuing to increase ( Goetz et al., 2005 [JoC] ). They also confirm other results about the effects of droughts ( Lotsch et al., 2005 [JoC] ), as well those made by ground measurements ( (D’Arrigo et al., 2004; ) Wilmking et al., 2004 [JoC] ).
Climate warming can also change the disturbance regime of forests by extending the range of some damaging insects, as observed during the last 20 years for bark beetles in the USA ( (Williams and Liebhold, 2002 ) ) or pine processionary moth in Europe ( (Battisti et al., 2005 ) ). The latter has displayed a northward shift of 27 km/decade near Paris, a 70 m/decade upward shift in altitude for southern slopes, and 30 m/decade for northern slopes in Italian mountains.
Trends in disturbance resulting from forest fires are still a subject of controversy. In spite of current management practices that tend to reduce fuel load in forests, climate variability is often the dominant factor affecting large wildfires, given the presence of ignition sources ( (McKenzie et al., 2004 ) ). This is confirmed by an analysis of forest fires in Siberia between 1989 and 1999 ( Conard et al., 2002 [JoC] ), which detected the significant impacts of two large fires in 1996 and 1998, resulting in 13 million ha burned and 14 to 20% of the annual global carbon emissions from forest fires. The increase in outdoor fires in England and Wales between 1965 and 1998 may be attributable to a trend towards warmer and drier summer conditions ( Cannell et al., 1999 [NPR, SRC] ). Repeated large forest fires during the warm season in recent years in the Mediterranean region and North Africa, as well as in California, have also been linked to drought episodes. One study of forest fires in Canada ( Gillett et al., 2004 [JoC] ) found that about half of the observed increase in burnt area during the last 40 years, in spite of improved fire-fighting techniques, is in agreement with simulated warming from a general circulation model (GCM). This finding is not fully supported by another study, which found that fire frequency in Canada has recently decreased in response to better fire protection and that the effects of climate change on fire activity are complex ( Bergeron et al., 2004 [MoS] ). However, it seems to be confirmed by another recent study ( Westerling et al., 2006 [JoC, ARC] ), which established a dramatic and sudden increase in large wildfire activity in the western USA in the mid- 1980 s closely associated with increased spring and summer temperatures and an earlier spring snow melt.
1.3.6.3 Summary of agriculture and forestry
Trends in individual climate variables or their combination into agro-climatic indicators show that there is an advance in phenology in large parts of North America and Europe, which has been attributed to recent regional warming. In temperate regions, there are clear signals of reduced risk of frost, longer growing season duration, increased biomass, higher quality (for grapevines, a climate-sensitive crop), insect expansion, and increased forest-fire occurrence that are in agreement with regional warming. These effects are hard to detect in aggregate agricultural statistics because of the influence of non-climate factors, particularly where advances in technology confound responses to warming. Although the present effects are of limited economic consequence and appear to lie within the ability of the sectors to adapt, both agriculture and forestry show vulnerability to recent extreme heat and drought events.
Table 1.11. Studies of the effects of weather and climate on human health.
| Health effect | Climate effect on health | Other driving forces | Study |
|---|---|---|---|
| Direct impacts of heat or cold | Temperature-related mortality in summers | Declining summer death rates due to air-conditioning adaptation | Diaz et al., 2002; Davis et al., 2003b; Beniston, 2004; Kysely, 2004 |
| Vector-borne diseases | Tick-borne encephalitis (TBE) increases in Sweden with milder climate | Increases in TBE may be due to changes in human and animal behaviour | Randolph, 2001 |
| High latitudinal spread of ticks – vectors for Lyme disease – with milder winters in Sweden and the Czech Republic | Lindgren et al., 2000; Danielová et al., 2006 | ||
| Food- and water-borne diseases | Salmonellosis in Australia associated with higher temperatures | E. coli and Cryptosporium outbreaks could not be attributed to climate change | D’Souza et al., 2004 Charron et al., 2004 |
| Pollen- and dust-related diseases | Increasing pollen abundance and allergenicity have been associated with warming climate | Pollen abundance also influenced by land-use changes | Levetin, 2001; Beggs, 2004 |
1.3.7 Human health
Here we evaluate evidence regarding observed changes in human health, important health exposures, and regional climate change. These observed changes are primarily related to temperature trends and changes in temperature extremes and relate to a range of infectious and non-infectious disease outcomes. These relationships are difficult to separate from the effects of major climate variability systems such as ENSO, which have been shown to be associated with the transmission and occurrence of diseases in certain locations ( (Kovats et al., 2003; ) Rodo et al., 2002 [JoC, SRC] ). Additionally, temperature and rainfall variability can be important determinants of the transmission of vector-borne diseases ( Githeko and Ndegwa, 2001 [MoS, SRC] ).
There is little evidence about the effects of observed climate change on health for two reasons: the lack of long epidemiological or health-related data series, and the importance of non-climate drivers in determining the distribution and intensity of human disease. Studies that have quantified the effect of climate or weather on health outcomes are listed in Table 1.11 . There is a wide range of driving forces that can affect and modify the impact of climate change on human health indicators. Consideration of reported trends in a given disease and the attribution to climate change needs to take into account three possible conditions.
1. That the change in disease incidence is real and due to changes in important non-climate determinants which include social factors, such as human population density and behaviour; housing facilities; public health facilities (e.g., water supply and general infrastructure, waste management and vector-control programmes); use of land for food, fuel and fibre supply; and results of adaptation measures (e.g., drug and insecticide use), as well as changed insecticide and drug resistance in pathogens and vector species ( Tillman et al., 2001 [JoC, MoS] ; Githeko and Woodward, 2003 [NPR, SRC] ;( Molyneux, 2003; ) ( Sutherst, 2004 ) ). Changes in land use and land cover can affect the local climate and ecosystems and should be considered when linking climate and health ( Patz et al., 2005 [JoC, ARC] ).
2. That the change in disease incidence is real and due to changes in climate factors, once all non-climate determinants have been considered and excluded as the main explanation (see, for example, ( Purse et al., 2006 ) ).
3. That the change in disease incidence is not real, but is only apparent due to changed reporting or may be due to changes in other apparent factors such as population growth or movement.
1.3.7.1 Effects of patterns in heat and cold stress
Episodes of extreme heat or cold have been associated with increased mortality ( (Huynen et al., 2001; ) Curriero et al., 2002 [ARC] ). There is evidence of recent increases in mean surface temperatures and in the number of days with higher temperatures, with the extent of change varying by region ( Karl and Trenberth, 2003 [PoC, JoC] ; Luterbacher et al., 2004 [JoC] ; Schär et al., 2004 [JoC] ; IPCC, 2007 [NPR, 2007] ). This increase in heatwave exposures, where heatwaves are defined as temperature extremes of short duration, has been observed in mid-latitudes in Europe and the USA. Individual events have been associated with excess mortality, particularly in the frail elderly, as was dramatically illustrated in the 2003 heatwave in western and central Europe, which was the hottest summer since 1500 ( Luterbacher et al., 2004 [JoC] ; Chapter 8 , Box 8.1 ).
In general, high-income populations have become less vulnerable to both heat and cold (see Chapter 8 , Section 8.2 ). Studies in Europe and in the USA of mortality over the past 30 to 40 years found evidence of declining death rates due to summer and winter temperatures ( (Davis et al., 2003a, ) b; ( Donaldson et al., 2003 ) ). Declines in winter mortality are apparent in many temperate countries primarily due to increased adaptation to cold ( Chapter 8 , Section 8.2.1.3 ( (Kunst et al., 1991; ) ( Carson et al., 2006 ) ). However, the mortality associated with extreme heatwaves has not declined. The 25,000 to 30,000 deaths attributed to the European heatwave is greater than that observed in the last century in Europe ( (Kosatsky, 2005 ) ). Analyses of long-term trends in heatwave-attributable (versus heat-attributable) mortality have not been undertaken.
1.3.7.2 Patterns in vector-borne diseases
Vector-borne diseases are known to be sensitive to temperature and rainfall (as shown by the ENSO effects discussed above). Consideration of these relationships suggests that warmer temperature is likely to have two major kinds of closely related, potentially detectable, outcomes: changes in vectors per se, and changes in vector-borne disease outcomes ( Kovats et al., 2001 [JoC, ARC] ). Insect and tick vectors would be expected to respond to changes in climate like other cold-blooded terrestrial species ( Table 1.9 ). There is some evidence that this is occurring in relation to disease vectors, but the evidence for changes in human disease is less clear.
Tick vectors
Changes in the latitudinal distribution and abundance of Lyme disease vectors in relation to milder winters have been well documented in high-latitude regions at the northern limit of the distribution in Sweden ( Lindgren et al., 2000 [NPR] ;( Lindgren and Gustafson, 2001 ) ), although the results may have been influenced by changes due to reporting and changes in human behaviour. An increase in TBE in Sweden since the mid- 1980 s is consistent with a milder climate in this period ( (Lindgren and Gustafson, 2001 ) ), but other explanations cannot be ruled out ( Randolph, 2001 [JoC] ).
Malaria
Since the TAR, there has been further research on the role of observed climate change on the geographical distribution of malaria and its transmission intensity in African highland areas but the evidence remains unclear. Malaria incidence has increased since the 1970 s at some sites in East Africa. Chen et al. 2006 [SRC] ) have demonstrated the recent spread of falciparum malaria and its vector Anopheles arabiensis in highland areas of Kenya that were malaria-free as recently as 20 years ago. It has yet to be proved whether this is due solely to warming of the environment. A range of studies have demonstrated the importance of temperature variability in malaria transmission in these highland sites ( Abeku et al., 2003 [SRC] ; Kovats et al., 2001 [JoC, ARC] ; Zhou et al., 2004 [JoC, SRC] ) (see Chapter 8 , Section 8.2.8.2 for a detailed discussion). While a few studies have shown the effect of long-term upward trends in temperature on malaria at some highland sites (e.g., Tulu, 1996 [NPR] ), other studies indicate that an increase in resistance of the malaria parasite to drugs, a decrease in vector-control activities and ecological changes may have been the most likely driving forces behind the resurgence of malaria in recent years. Thus, while climate is a major limiting factor in the spatial and temporal distribution of malaria, many non-climatic factors (drug resistance and HIV prevalence, and secondarily, cross-border movement of people, agricultural activities, emergence of insecticide resistance, and the use of DDT for indoor residual spraying) may alter or override the effects of climate ( (Craig et al., 2004; ) ( Barnes et al., 2005 ) ).
There is a shortage of concurrent detailed and long-term historical observations of climate and malaria. Good-quality time-series of malaria records in the East African and the Horn of Africa highlands are too short to address the early effects of climate change. Very few sites have longer data series, and the evidence on the role of climate change is unresolved ( Hay et al., 2002a [JoC] , 2002b; Patz et al., 2002 [JoC, SRC] ;( Shanks et al., 2002 ) ), although a recent study has confirmed warming trends at these sites ( Pascual et al., 2006 [JoC, SRC] ).
1.3.7.3 Emerging food- and water-borne diseases
Food- and water-borne diseases (WBD) are major adverse conditions associated with warming and extreme precipitation events. Bacterial infectious diseases are sometimes sensitive to temperature, e.g., salmonellosis ( (D’Souza et al., 2004 ) ), and WBD outbreaks are sometimes caused by extreme rainfall ( Casman et al., 2001 [JoC] ; Curriero et al., 2001 [ARC] ; Rose et al., 2002 [Ambiguous] ;( Charron et al., 2004; ) ( Diergaardt et al., 2004 ) ) but, again, no attribution to longer-term trends in climate has been attempted.
The impact on health of dust and dust storms has not been well described in the literature. Dust related to African droughts has been transported across the Atlantic to the Caribbean ( Prospero and Lamb, 2003 [JoC] ), while a dramatic increase in respiratory disease in the Caribbean has been attributed to increases in Sahara dust, which has in turn, been linked to climate change ( (Gyan et al., 2003 ) ).
1.3.7.4 Pollen- and dust-related diseases
There is good evidence that observed climate change is affecting the timing of the onset of allergenic pollen production. Studies, mostly from Europe, indicate that the pollen season has started earlier (but later at high latitudes) in recent decades, and that such shifts are consistent with observed changes in climate. The results concerning pollen abundance are more variable, as pollen abundance can be more strongly influenced by land-use changes and farming practices ( (Teranishi et al., 2000; ) ( Rasmussen, 2002; ) van Vliet et al., 2002 [JoC] ;( Emberlin et al., 2003; ) WHO, 2003 [NPR] ; Beggs, 2004 [MoS, ARC] ; Beggs and Bambrick, 2005 [ARC] ) (see Section 1.3.5 ). There is some evidence that temperature changes have increased pollen abundance or allergenicity ( Beggs, 2004 [MoS, ARC] ) (see Chapter 8 , Section 8.2.7 ). Changing agricultural practices, such as the replacement of haymaking in favour of silage production, have also affected the grass-pollen season in Europe.
1.3.7.5 Summary of human health
There is now good evidence of changes in the northward range of some disease vectors, as well as changes in the seasonal pattern of allergenic pollen. There is not yet any clear evidence that climate change is affecting the incidence of human vector-borne diseases, in part due to the complexity of these disease systems. High temperature has been associated with excess mortality during the 2003 heatwave in Europe. Declines in winter mortality are apparent in many temperate countries, primarily due to increased adaptation to cold.
Rapid-onset meteorological hazards with the potential to cause the greatest destruction to property and lives include extreme river floods, intense tropical and extra-tropical cyclone windstorms (along with their associated coastal storm surges), as well as the most severe supercell thunderstorms. Here we assess the evidence for a change in the frequency, geography and/or severity of these high-energy events. By definition, the extreme events under consideration here are rare events, with return periods at a specific location typically in excess of 10 to 20 years, as the built environment is generally sited and designed to withstand the impacts of more frequent extremes. Given that the strong rise in global temperatures only began in the 1970 s, it is difficult to demonstrate statistically a change in the occurrence of extreme floods and storms (with return periods of 20 years or more) simply from the recent historical record ( Frei and Schar, 2001 [JoC] ). In order to identify a change in extreme flood and storm return periods, data has been pooled from independent and uncorrelated locations that share common hazard characteristics so as to search collectively for changes in occurrence. A search for a statistically significant change in occurrence characteristics of relatively high-frequency events (with return periods less than 5 years) can also be used to infer changes at longer return periods.
1.3.8 Disasters and hazards
1.3.8.1 Extreme river floods
The most comprehensive available global study examined worldwide information on annual extreme daily flows from 195 rivers, principally in North America and Europe, and did not find any consistent trends, with the number of rivers showing statistically significant increases in annual extreme flows being approximately balanced by the number showing a decrease ( Kundzewicz, 2004 [NPR, ARC] ) (see Section 1.3.2.2 ). However, in terms of the most extreme flows, when data were pooled across all the rivers surveyed in Europe, a rising trend was found in the decade of the maximum observed daily flow, with four times as many rivers showing the decade of highest flow in the 1990 s rather than in the 1960 s.
Again, with a focus only on the most extreme flows, a pooled study examined great floods with return periods estimated as greater than 100 years on very large rivers (with catchments greater than 200,000 km2) in Asia, North America, Latin America, Europe and Africa ( Milly et al., 2002 [JoC] ). From the pooled record of all the rivers, the observed trend in the population of 100-year flood events, at a 95% confidence interval averaged across all basins, has been positive since the Mississippi floods in 1993 and can be detected intermittently since 1972 . Analysis of available long-term river flow records shows that since 1989 more than half of Scotland’s largest rivers (notably those draining from the west) have recorded their highest flows ( Werrity et al., 2002 [NPR] ). Of sixteen rivers surveyed, with a median record of thirty-nine years, eight had their maximum flow during the period 1989 to 1997, a period of high NAO index (based on the pressure difference between Iceland and the Azores) values consistent with storm tracks bringing high levels of precipitation to the northern UK.
1.3.8.2 North-east Atlantic extra-tropical cyclones
The North-east Atlantic is the region with the deepest observed central pressures of extra-tropical cyclones, and the adjacent margin of north-west Europe has the greatest levels of extra-tropical cyclone historical building damage, forestry windthrow, and storm-surge impacts observed worldwide. Many studies report an increase in the 1980 s in the number of deep (and high wind-speed) extra-tropical cyclone storms in this region (see ( Günther et al., 1998 ) ) returning to levels not previously seen since the late 19th century (see Trenberth et al., 2007 [NPR, PoC, 2007] , Section 3.5.3). Various measures, including increases in the number of deep storms (with central pressures less than 970 hPa) and reductions in the annual pressure minimum of storms crossing the Greenwich Meridian all show evidence for intensification, in particular between 1980 and 1993, when there were a series of major damaging storms. In the North-east Atlantic, wave heights showed significant increases over the period from 1970 to 1995 ( Woolf et al., 2002 [MoS] ) in parallel with the NAO index, which reached its highest values ever (reflecting deep low pressure over Iceland) in the years of 1989 to 1990 . Intense storms returned at the end of the 1990 s, when there were three principal damaging storms across western Europe in December 1999 . However, since that time, as winter NAO values have continued to fall (through to March,. However, since that time, as winter NAO values have continued to fall (through to March,), there has been a significant decline in the number of deep and intense storms passing into Europe, to some of the lowest levels seen for more than 30 years. (Other high-latitude regions of extra-tropical cyclone activity also show variations without simple trends: see Trenberth et al., 2007 [NPR, PoC, 2007] , Section 3.5.3.)
While other basins do not show overall increases in activity, observations based on satellite observations of intensity (which start in the 1970 s) suggest a shift in the proportion of tropical cyclones that reached the higher intensity (CAT 4 and CAT 5) from close to 20% of the total in the 1970 s rising to 35% since the 1990 s ( Webster et al., 2005 [JoC] ). Although challenged by some climatologists based on arguments of observational consistency, as quoted from Trenberth et al., 2007 [NPR, PoC, 2007] ) Section 3.8.3) “the trends found by Emanuel 2005 [JoC] and Webster et al. 2005 [JoC] ) appear to be robust in strong association with higher SSTs”. Increases in the population of intense hurricanes in 2005 created record catastrophe losses, principally in the Gulf Coast, USA, and in Florida, when a record four Saffir-Simpson severe (CAT 3-5) hurricanes made landfall, causing more than US$100 billion in damages with almost 2,000 fatalities.
1.3.8.3 Tropical cyclones
While overall numbers of tropical cyclones worldwide have shown little variation over the past 40 years ( Pielke et al., 2005 [JoC] ), there is evidence for an increase in the average intensity of tropical cyclones in most basins of tropical cyclone formation since 1970 ( Webster et al., 2005 [JoC] ) as well as in both the number and intensity of storms in the Atlantic ( Emanuel, 2005 [JoC] ), the basin with the highest volatility in tropical cyclone numbers (see Trenberth et al., 2007 [NPR, PoC, 2007] , Sections 3.8.3 and 3.8.3.2).
Although the Atlantic record of hurricanes extends back to 1851, information on tracks is only considered comprehensive after 1945 and for intensity assessments it is only complete since the 1970 s ( Landsea, 2005 [JoC] ). From 1995 to 2005, all seasons were above average in the Atlantic, with the exception of the two El Niño years of 1997 and 2002, when activity was suppressed – as in earlier El Niños. The number of intense (CAT 3-5 on the Saffir-Simpson Hurricane Scale) storms in the Atlantic since 1995 was more than twice the level of the 1970 to 1994 period, and 2005 was the most active year ever for Atlantic hurricanes on a range of measures, including number of hurricanes and number of the most intense CAT 5 hurricanes.
In the Atlantic, among the principal reasons for the increases in activity and intensity ( Chelliah and Bell, 2004 [JoC] ) are trends for increased sea surface temperatures in the tropical North Atlantic. The period since 1995 has had the highest temperatures ever observed in the equatorial Atlantic – “apparently as part of global warming” (see Trenberth et al., 2007 [NPR, PoC, 2007] , Section 3.8.3.2). The first and only tropical cyclone ever identified in the South Atlantic occurred in March, Section 3.8.3.2). The first and only tropical cyclone ever identified in the South Atlantic occurred in March,.
1.3.8.4 Economic and insurance losses
Economic losses attributed to natural disasters have increased from US$75.5 billion in the 1960 s to US$659.9 billion in the 1990 s (a compound annual growth rate of 8%) (United Nations Development Programme, 2004 ). Private-sector data on insurance costs also show rising insured losses over a similar period (Munich Re Group, 2005; Swiss Reinsurance Company, 2005 ). The dominant signal is of significant increase in the values of exposure ( Pielke and Hoppe, 2006 [NPR, MoS] ).
However, as has been widely acknowledged, failing to adjust for time-variant economic factors yields loss amounts that are not directly comparable and a pronounced upward trend through time for purely economic reasons. A previous normalisation of losses, undertaken for U.S. hurricanes by ( Pielke and Landsea 1998 ) ) and U.S. floods ( Pielke et al., 2002 [NPR, MoS] ) included normalising the economic losses for changes in wealth and population so as to express losses in constant dollars. These previous national U.S. assessments, as well as those for normalised Cuban hurricane losses ( Pielke et al., 2003 [ARC] ), did not show any significant upward trend in losses over time, but this was before the remarkable hurricane losses of 2004 and 2005 .
A global catalogue of catastrophe losses was constructed ( Muir Wood et al., 2006 [NPR, MoS] ), normalised to account for changes that have resulted from variations in wealth and the number and value of properties located in the path of the catastrophes, using the method of Landsea et al. 1999 [JoC] ). The global survey was considered largely comprehensive from 1970 to 2005 for countries and regions (Australia, Canada, Europe, Japan, South Korea, the USA, Caribbean, Central America, China, India and the Philippines) that had centralised catastrophe loss information and included a broad range of peril types: tropical cyclone, extra-tropical cyclone, thunderstorm, hailstorm, wildfire and flood, and that spanned high- and low-latitude areas.
Once the data were normalised, a small statistically significant trend was found for an increase in annual catastrophe loss since 1970 of 2% per year (see Supplementary Material Figure SM1.1). However, for a number of regions, such as Australia and India, normalised losses show a statistically significant reduction since 1970 . The significance of the upward trend is influenced by the losses in the USA and the Caribbean in 2004 and 2005 and is arguably biased by the relative wealth of the USA, particularly relative to India.
1.3.8.5 Summary of disasters and hazards
Global losses reveal rapidly rising costs due to extreme weather-related events since the 1970 s. One study has found that while the dominant signal remains that of the significant increases in the values of exposure at risk, once losses are normalised for exposure, there still remains an underlying rising trend. For specific regions and perils, including the most extreme floods on some of the largest rivers, there is evidence for an increase in occurrence.
The literature on observed changes in socio-economic indicators in response to recent climate change is sparse. Here we summarise some of the few examples related to energy demand and tourism, and some studies on regional adaptations to climate trends. Other relevant indicators include energy supply and markets for natural resources (e.g., timber, fisheries). Indicators of adaptation such as domestic insurance claims, energy demand, and changes in tourism are being defined and tracked for the UK and Europe (Defra, 2003; EEA, 2004 [NPR] ).
1.3.9 Socio-economic indicators
1.3.9.1 Energy demand
Buildings account for a significant part of total energy use, up to 50% in some developed countries ( (Lorch, 1990; ) also see Levine et al., 2007 [NPR, 2007] ), and the design and energy performance of buildings are related to climate ( (Steemers, 2003 ) ). Work related to climate change and building energy use can be grouped into two major areas – weather data analysis and building energy consumption.
Weather data analysis
A study on 1981 to 1995 weather data ( (Pretlove and Oreszczyn, 1998 ) ) indicated that temperature and solar radiation in the London area (UK) had changed significantly over the period, and climatic data used for energy design calculations could lead to 17% inaccuracies in building energy-use estimates. Based on 1976 to 1995 temperature data from 3 key UK sites, ( Levermore and Keeble 1998 ) ) found that the annual mean dry-bulb temperature had increased by about 1°C over the 19-year period, with milder winters and warmer summers. In sub-tropical Hong Kong SAR, the 40-year period ( 1961 to 2000 ) weather data showed an underlying trend of temperature rise, especially during the last 10 years ( 1991 to 2000 ( Lam et al., 2004 [SRC] ). The increases occurred largely during the winter months and the impact on peak summer design conditions and cooling requirements, and hence energy use, was considered insignificant. In the 1990 s and 2000 s, many countries experienced extreme phenomena (notably heatwaves in summer), which induced exceptional peaks of electric power consumption ( Tank and Konnen, 2003 [JoC] ). These had notable impacts on human mortality ( Section 1.3.7 ) and local socio-economic systems ( Easterling et al., 2000 [PoC, JoC, MoS, ARC] ; Parmesan et al., 2000 [JoC, SRC] ; Johnson et al., 2004 [ARC] ). Two well-documented cases are the heatwaves in Chicago in 1995 ( Karl and Knight, 1997 [JoC] ) and in Europe in 2003 ( Schär et al., 2004 [JoC] ; Trigo et al., 2005 [JoC] ).
Building energy consumption
One example related to energy and climate concerns cooling during hot weather. Energy use has been and will continue to be affected by climate change, in part because air-conditioning, which is a major energy use particularly in developed countries, is climate-dependent. However, the extent to which temperature rise has affected energy use for space heating/cooling in buildings is uncertain. There is a concern that energy consumption will increase as air-conditioning is adopted for warmer summers (see Levine et al., 2007 [NPR, 2007] ). It is likely that certain adaptation strategies (e.g., tighter building energy standards) have been (or would be) taken in response to climate change (e.g., ( Camilleri et al., 2001; ) ( Larsson, 2003; ) ( Sanders and Phillipson, 2003; ) ( Shimoda, 2003 ) ). Adaptation strategies and implementation are strongly motivated by the cost of energy. Besides, in terms of thermal comfort, there is also the question of people adapting to warmer climates (e.g., de Dear and Brager, 1998 [MoS] ;( Nicol, 2004 ) ).
1.3.9.2 Tourism
Climate is a major factor for tourists when choosing a destination ( Aguiló et al., 2005 [MoS] ) and both tourists and tourism stakeholders are sensitive to fluctuations in the weather and climate ( Wall, 1998 [JoC] ). Statistical analyses by Maddison 2001 [JoC] Lise and Tol 2002 [JoC, ARC] and Hamilton 2003a [NPR] ), and a simulation study ( Hamilton et al., 2003 [NPR, MoS, ARC] ), have shown the relevance of climatic factors as determinants of tourist demand, next to economic and political conditions, fashion, media attention, and environmental quality. As a result of the complex nature of the interactions that exist between tourism, the climate system, the environment and society, it is difficult to isolate the direct observed impacts of climate change upon tourism activity. There is sparse literature about this relationship at any scale. Responses in skiing have been documented in Switzerland, Austria, the eastern USA and Chile ( OECD, 2007 [NPR, 2007] ;( Elsasser and Messerli, 2001; ) Steininger and Weck-Hannemann, 2002 [NotFound] ; Beniston, 2003 [JoC, ARC] , 2004; Casassa et al., 2003 [NPR, SRC] ; Hamilton et al., 2005 [JoC, MoS, ARC] ) (see Section 1.3.1.1 ).
Changes in socio-economic activities and modes of human response to climate change, including warming, are just beginning to be systematically documented in the cryosphere ( MacDonald et al., 1997 [NPR] ; Krupnik and Jolly, 2002 [NPR] ; Huntington and Fox, 2004 [NPR] ; Community of Arctic Bay et al., 2005 ). The impacts associated with these changes are both positive and negative, and are most pronounced in relation to the migration patterns, health and range of animals and plants that indigenous groups depend on for their livelihood and cultural identity. Responses vary by community and are dictated by particular histories, perceptions of change and the viability of options available to groups ( Ford and Smit, 2004 [ARC] ; Helander and Mustonen, 2004 [NPR] ). In Sachs Harbour, Canada, responses include individual adjustments to the timing, location and methods of harvesting animals, as well as adjusting the overall mix of animals harvested to minimise risk ( (Berkes and Jolly, 2002 ) ). Communities that are particularly vulnerable to coastal erosion such as Shishmeref, Alaska, are faced with relocation. Many communities in the North are stepping up monitoring efforts to watch for signs of change so they can respond accordingly in both the long and short term ( Fox, 2002 [NPR] ). Agent-based simulation models (i.e., models dealing with individual decision making and interactions among individuals) are also being developed to assess adaptation and sustainability in small-scale Arctic communities ( Berman et al., 2004 [MoS] ). Effective responses will be governed by increased collaboration between indigenous groups, climate scientists and resource managers ( Huntington and Fox, 2004 [NPR] ).
1.3.9.3 Regional adaptation
There are several studies that show societies adapting to climate changes such as drying trends or increasing temperatures (see Chapter 17 ). For example, responses to recent historical climate variability and change in four locations in southern Africa demonstrated that people were highly aware of changes in the climate, including longer dry seasons and more uncertain rainfall, and were adjusting to change through collective and individual actions that included both short-term coping through switching crops and long-term adaptations such as planting trees, and commercialising and diversifying livelihoods ( Thomas and Twyman, 2005 [JoC] ; Thomas et al., 2005 [NPR] ). One of the most striking conclusions was the importance of local institutions and social capital such as farming associations in initiating and supporting adaptations. The use of climate science in adapting to water management during a long-term drought has been documented in Western Australia ( Power et al., 2005 [JoC] ).
In Europe, evidence is also accumulating that people are adapting to climate change, either in response to observed changes or in anticipation of predicted change. For example, in the UK, a large number of adaptations have been identified including changes in flood management guidelines (assuming more extremes), hiring of climate change managers, changing nature conservation and disaster plans, climate-proofing buildings, planting different crops and trees, and converting a skiing area to a walking centre in Scotland ( West and Gawith, 2005 [NPR] ).
1.4 Larger-scale aggregation and attribution to anthropogenic climate change
Larger-scale aggregation offers insights into the relationships between the observed changes assessed in Section 1.3 and temperature, by combining results from many studies over multiple systems and larger regions. Aggregation through meta-analysis is described next, followed by joint attribution through climate model studies, and synthesis of the observed changes described in Section 1.3 .
1.4.1 Larger-scale aggregation
This section evaluates studies that use techniques that aggregate from individual observations at sites to regional, continental and global scales. Meta-analysis is a statistical method of combining quantitative findings from many studies investigating similar factors for the purpose of finding a general result. The methods used in the various studies, however, need not be similar. The criteria for inclusion of studies in a meta-analysis are determined a priori, and rigorously followed to avoid investigator effect.
Several studies have examined the ‘fingerprint’ of observed warming in recent decades on the phenology and distribution of plants and animal species using meta-analyses ( Root and Schneider, 2002 [NPR, PoC, SRC] ; Parmesan and Yohe, 2003 [JoC, ARC] ; Root et al., 2003 [PoC, JoC, SRC] ). Although the detailed results of these studies are different, because they used different species and different methods, they all conclude that a significant impact of warming is already discernible in animal and plant populations at regional and continental scales in the Northern Hemisphere.
One meta-analysis ( Parmesan and Yohe, 2003 [JoC, ARC] ) of 31 studies of more than 1,700 species showed that recent biological trends matched the expected responses to warming. They estimated northward range shifts of 6.1 km/decade for northern range boundaries of species living in the Northern Hemisphere and advancement of spring events in Northern Hemisphere species by 2.3 days/decade. They also defined a diagnostic fingerprint of temporal and spatial ‘sign-switching’ responses uniquely predicted by 20th-century observed climate trends. Among long-term, large-scale, multi-species data sets, this diagnostic fingerprint was found for 279 species. They concluded, with ‘very high confidence’, that climate change is already affecting living systems.
After examining over 2,500 articles on climate change and a wide array of species from around the globe, another study found that 143 studies fitted the criteria for inclusion in their meta-analyses ( Root et al., 2003 [PoC, JoC, SRC] ). They focused on only those species showing a significant change and found that about 80% of the species showing change were changing in the direction expected with warming. The types of changes included species expanding their ranges polewards and higher in elevation, and advances in the timing of spring events by about 5 days/decade over the last 30 years. This number is larger than the 2.3 days/decade found by Parmesan and Yohe 2003 [JoC, ARC] ), because those authors included both changing and not-changing species in their analysis, while Root and co-authors only included changing species. A more recent meta-analysis of bird arrival dates ( Lehikoinen et al., 2004 [SRC] ) showed strong evidence of earlier arrival. Of 983 data series, 39% were significantly earlier and only 2% significantly later for first arrival dates.
The EU COST725 network analysis project had as its main objective the establishment of a comprehensive European reference data set of phenological observations that could be used for climatological purposes, particularly climate monitoring and the detection of changes (see Box 1.3 ).
Box 1.3. Phenological responses to climate in Europe: the COST725 project
The COST725 meta-analysis project used a very large phenological network of more than 125,000 observational series of various phases in 542 plant and 19 animal species in 21 European countries, for the period 1971 to 2000 . The time-series were systematically (re-)analysed for trends in order to track and quantify phenological responses to changing climate. The advantage of this study is its inclusion of multiple verified nationally reported trends at single sites and/or for selected species, which individually may be biased towards predominant reporting of climate-change-induced impacts. Overall, the phenology of the species (254 national series) was responsive to temperature of the preceding month, with spring/summer phases advancing on average by 2.5 days/°C and leaf colouring/fall being delayed by 1.0 day/°C.
The aggregation of more than 100,000 trends revealed a clear signal across Europe of changing spring phenology with 78% of leaf unfolding and flowering records advancing (31% significantly (sig.)) and only 22% delayed (3% sig.) ( Figure 1.6 ). Fruit ripening was mostly advanced (75% advancing, 25% sig.; 25% delayed, 3% sig.). The signal in farmers’ activities was generally smaller (57% advancing, 13% sig.; 43% delayed, 6% sig.). Autumn trends (leaf colouring/fall) were not as strong. Spring and summer exhibited a clear advance by 2.5 days/decade in Europe, mean autumn trends were close to zero, but suggested more of a delay when the average trend per country was examined (1.3 days/decade).
The patterns of observed changes in spring (leafing, flowering and animal phases) were spatially consistent and matched measured national warming across 19 European countries (correlation = -0.69, P < 0.001); thus the phenological evidence quantitatively mirrors regional climate warming. The COST725 results assessed the possible lack of evidence at a continental scale as 20%, since about 80% of spring/summer phases were found to be advancing. The findings strongly support previous studies in Europe, confirming them as free from bias towards reporting global climate change impacts ( Menzel et al., 2006b [JoC, SRC] ).
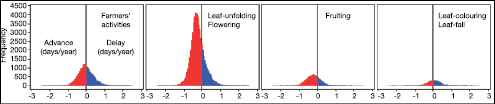
Figure 1.6. Frequency distributions of trends in phenology (in days/year) over 1971 to 2000 for 542 plant species in 21 European countries. From Menzel et al. 2006b [JoC, SRC] ).
1.4.2 Joint attribution
Joint attribution involves attribution of significant changes in a natural or managed system to regional temperature changes, and attribution of a significant fraction of the regional temperature change to human activities. This has been performed using studies with climate models to assess observed changes in several different physical and biological systems. An assessment of the relationship between significant observed changes from Section 1.3 and significant regional temperature changes is presented in Section 1.4.2.3 .
1.4.2.1 Attributing regional temperature change
It is likely that there has been a substantial anthropogenic contribution to surface temperature increases averaged over each continent except Antarctica since the middle of the 20th century ( Hegerl et al., 2007 [NPR, ARC, 2007] , Section 9.4.2). Statistically significant regional warming trends over the last 50 and 30 years are found in many regions of the globe ( Spagnoli et al., 2002 [NPR, JoC] ; Karoly and Wu, 2005 [JoC, SRC] ; Karoly and Stott, 2006 [SRC] ; Knutson et al., 2006 [JoC, MoS] ; Zhang et al., 2006 [JoC, MoS] ; Trenberth et al., 2007 [NPR, PoC, 2007] , Figure 3.9). These warming trends are consistent with the response to increasing greenhouse gases and sulphate aerosols and likely cannot be explained by natural internal climate variations or the response to changes in natural external forcing (solar irradiance and volcanoes).
Attributing temperature changes on smaller than continental scales and over time-scales of less than 20 years is difficult due to low signal-to-noise ratios at those scales. Attribution of the observed warming to anthropogenic forcing is easier at larger scales because averaging over larger regions reduces the natural variability more, making it easier to distinguish between changes expected from different external forcings, or between external forcing and climate variability.
The influence of anthropogenic forcing has also been detected in various physical systems over the last 50 years, including increases in global oceanic heat content, increases in sea level, shrinking of alpine glaciers, reductions in Arctic sea ice extent, and reductions in spring snow cover ( Hegerl et al., 2007 [NPR, ARC, 2007] ).
1.4.2.2 Joint attribution using climate model studies
Several studies have linked the observed responses in some biological and physical systems to regional-scale warming due to anthropogenic climate change using climate models.
One study demonstrated joint attribution by considering changes in wild animals and plants ( Root et al., 2005 [PoC, JoC, SRC] ). They found spring phenological data for 145 Northern Hemisphere species from 31 studies. The changes in the timing of these species’ spring events (e.g., blooming) are significantly associated with the changes in the actual temperatures recorded as near to the study site as possible and for the same years that the species were observed. If the temperature was warming and the species phenology was getting earlier in the year, then the expected association would be negative, which is what was found for the correlations between the species data and the actual temperatures ( Figure 1.7 ).
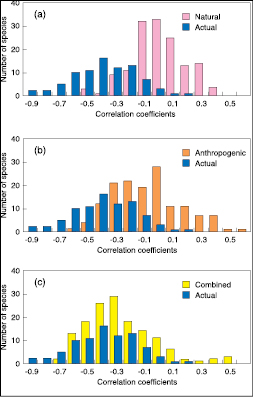
Figure 1.7. Plotted are the frequencies of the correlation coefficients (associations) between the timing of changes in traits (e.g., earlier egg-laying) of 145 species and modelled (HadCM3) spring temperatures for the grid-boxes in which each species was examined. At each location, all of which are in the Northern Hemisphere, the changing trait is compared with modelled temperatures driven by: (a) natural forcings (purple bars), (b) anthropogenic (i.e., human) forcings (orange bars), and (c) combined natural and anthropogenic forcings (yellow bars). In addition, on each panel the frequencies of the correlation coefficients between the actual temperatures recorded during each study and changes in the traits of 83 species, the only ones of the 145 with reported local-temperature trends, are shown (dark blue bars). On average the number of years that species were examined is about 28, with average starting and ending years of 1960 and 1998 . Note that the agreement: (a) between the natural and actual plots is weaker (K = 60.16) than (b) between the anthropogenic and actual (K = 35.15), which in turn is weaker than( c) the agreement between combined and actual (K = 3.65). Taken together, these plots show that a measurable portion of the warming regional temperatures to which species are reacting can be attributed to humans, therefore showing joint attribution (after Root et al., 2005 [PoC, JoC, SRC] ).
Temperature data from the HadCM3 climate model were used to determine whether the changes in the actual temperatures with which the phenological changes in species were associated were due to human or natural causes. Modelled temperature data were derived for each species, over the same years a species was studied and for the grid box within which the study area was located. Three different forcings were used when calculating the modelled values: natural only, anthropogenic only, and combined natural and anthropogenic. Each species’ long-term phenological record was correlated with the three differently forced temperatures derived for the location where the species was recorded. The agreement is quite poor between the phenological changes in species and modelled temperatures derived using only natural climatic forcing (K = 60.16, P > 0.05; Figure 1.7 a). A stronger agreement occurs between the same phenological changes in species and temperatures modelled using only anthropogenic forcing (K = 35.15, P > 0.05; Figure 1.7 b). As expected, the strongest agreement is with the modelled temperatures derived using both natural and anthropogenic (combined) forcings (K = 3.65, P < 0.01; Figure 1.7 c). While there is uncertainty in downscaling the model-simulated temperature changes to the areas that would affect the species being examined, these results demonstrate some residual skills, thereby allowing joint attribution to be shown.
Other similar studies have shown that the retreat of two glaciers in Switzerland and Norway cannot be explained by natural variability of climate and the glaciers alone ( Reichert et al., 2002 [JoC] ), that observed global patterns of changes in streamflow are consistent with the response to anthropogenic climate change ( Milly et al., 2005 [JoC] ), and that the observed increase in the area of forests burned in Canada over the last four decades is consistent with the response due to anthropogenic climate change ( Gillett et al., 2004 [JoC] ). Each of these studies has its limitations for joint attribution. For example, the analysis by Reichert used a climate model linked to a local glacier mass balance model through downscaling and showed that the observed glacier retreat over the 20th century could not be explained by natural climate variability. However, they did not show that the observed retreat was consistent with the response to anthropogenic climate change, nor did they eliminate other possible factors, such as changes in dust affecting the albedo of the glacier. Similarly, Gillett and colleagues showed that the observed increases in area of forests burned was consistent with the response to anthropogenic forcing and not consistent with natural climate variability. However, they did not consider changes in forest management as a factor, nor did they consider the climate response to other external forcing factors.
Taken together, these studies show a discernible influence of anthropogenic climate change on specific physical (cryosphere, hydrology) and biological (forestry and terrestrial biology) systems.
1.4.2.3 Synthesis of studies
Next, a synthesis of the significant observed changes described in Section 1.3 and the observed regional temperatures over the last three decades was performed. Significant observed changes documented since the TAR were divided into the categories of cryosphere, hydrology, coastal processes, marine and freshwater biological systems, terrestrial biological systems, and agriculture and forestry, as assessed in Section 1.3 . Studies were selected that demonstrate a statistically significant trend in change in systems related to temperature or other climate change variable as described by the authors, for the period 1970 to 2004 (study periods may be extended later), with at least 20 years of data. Observations in the studies are characterised as ‘change consistent with warming’ and ‘change not consistent with warming’.
Figure 1.8 shows the warming trends over the period 1970 to 2004 (from the GHCN-ERSST dataset; Smith and Reynolds, 2005 [JoC] ) and the geographical locations of significant observed changes. A statistical comparison shows that the agreement between the regions of significant and regional warming across the globe and the locations of significant observed changes in systems consistent with warming is very unlikely to be due to natural variability in temperatures or natural variability in the systems ( Table 1.12 ) (see also Supplementary Material).
For regions where there are both significant warming and observed changes in systems, there is a much greater probability of finding coincident significant warming and observed responses in the expected direction. The statistical agreement between the patterns of observed significant changes in systems and the patterns of observed significant warming across the globe very likely cannot be explained by natural climate variability.
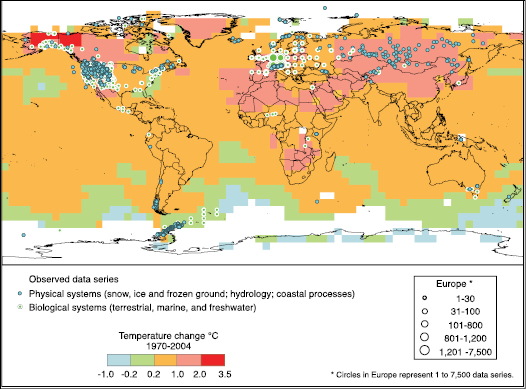
Figure 1.8. Locations of significant changes in observations of physical systems (snow, ice and frozen ground; hydrology; coastal processes) and biological systems (terrestrial, marine and freshwater biological systems), are shown together with surface air temperature changes over the period 1970 to 2004 (from the GHCN-ERSST datatset). The data series met the following criteria: (1) ending in 1990 or later; (2) spanning a period of at least 20 years; (3) showing a significant change in either direction, as assessed by individual studies. White areas do not contain sufficient observational climate data to estimate a temperature trend.
Uncertainties in observed change studies at the regional level relate to potential mismatches between climate and system data in temporal and spatial scales and lack of time-series of sufficient length to determine whether the changes are outside normal ranges of variability. The issue of non-climate driving forces is also important. Land-use change, changes in human management practices, pollution and demography shifts are all, along with climate, drivers of environmental change. More explicit consideration of these factors in observed change studies will strengthen the robustness of the conclusions. However, these factors are very unlikely to explain the coherent responses that have been found across the diverse range of systems and across the broad geographical regions considered ( Figure 1.9 ).
Since systems respond to an integrated climate signal, precise assignment of the proportions of natural and anthropogenic forcings in their responses in a specific grid cell is difficult. The observed continent-averaged warming in all continents except Antarctica over the last 50 years has been attributed to anthropogenic causes ( IPCC, 2007 [NPR, 2007] , Summary for Policy Makers). The prevalence of observed changes in physical and biological systems in expected directions consistent with anthropogenic warming on every continent and in some oceans means that anthropogenic climate change is having a discernible effect on physical and biological systems at the global scale.
Table 1.12. Global comparison of significant observed changes in physical and biological systems with regional temperature changes. Fraction of 5°*5° cells with significant observed changes in systems (from studies considered in this chapter) and temperature changes (for 1970 to 2004 from the GHCN-ERSST dataset ( Smith and Reynolds, 2005 [JoC] )) in different categories (significant warming, warming, cooling, significant cooling). Expected values shown in parentheses are for the null hypotheses:
| Temperature cells | Cells with significant observed change consistent with warming | Cells with significant observed change not consistent with warming |
|---|---|---|
| Significant warming | 50% (2.5%) | 7% (2.5%) |
| Warming | 34% (22.5%) | 6% (22.5%) |
| Cooling | 3% (22.5%) | 0% (22.5%) |
| Significant cooling | 0% (2.5%) | 0% (2.5%) |
| Chi-squared value (significance level) | 369 (<<1%) |
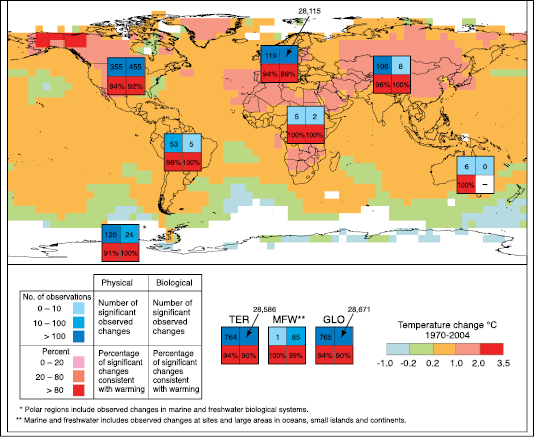
Figure 1.9. Changes in physical and biological systems and surface temperature. Background shading and the key at the bottom right show changes in gridded surface temperatures over the period 1970 to 2004 (from the GHCN-ERSST dataset). The 2*2 boxes show the total number of data series with significant changes (top row) and the percentage of those consistent with warming (bottom row) for (i) continental regions; North America, Latin America, Europe, Africa, Asia, Australia and New Zealand, and Polar Regions; and (ii) global-scale: Terrestrial (TER), Marine and Freshwater (MFW), and Global (GLO). The numbers of studies from the seven regional boxes do not add up to the global totals because numbers from regions except Polar do not include the numbers related to Marine and Freshwater systems. White areas do not contain sufficient observational climate data to estimate a temperature trend.
1.5 Learning from observed responses: vulnerability, adaptation and research needs
The great majority of observed changes are consistent with functional understanding and modelled predictions of climate impacts. Examples of expected responses include infrastructure effects of melting in the cryosphere, effects of intensifying droughts and runoff, and effects of rising sea levels. In marine, freshwater and terrestrial biological systems, changes in morphology, physiology, phenology, reproduction, species distribution, community structure, ecosystem processes and species evolutionary processes are, for the most part, in the predicted directions. Agricultural crops have shown similar trends in phenology, and management practices along with the spread of pests and diseases coincide with expected responses to warming. Responses of yields in the few crops with reported changes coincide with model predictions. Temperature-sensitive vectors, e.g., ticks, have spread for some human diseases.
Observed changes are prevalent across diverse physical and biological systems and less prevalent in managed systems and across many, but not all, geographical regions. While there is evidence of observed changes in every continent, including Antarctica, much evidence comes from studies of observed changes in Northern Hemisphere mid- and high latitudes and often from higher altitudes. Significant evidence comes from high-latitude waters in the Northern Hemisphere as well. Evidence is primarily found in places where warming is most pronounced. Documentation of observed changes in tropical regions is still sparse.
The evidence for adaptation and vulnerability to observed climate change is most prevalent in places where warming has been the greatest and in systems that are more sensitive to temperature. Thus, documented changes relating to adaptation in the Arctic and mountain regions include reduced outdoor and tourism activities, and alterations in indigenous livelihoods in the Arctic. Responses to climate change, including warming, vary by community and are beginning to be systematically documented ( Section 1.3.9 ).
In terrestrial biological systems, special conservation measures by resource managers are carried out as an adaptation to the impacts of climate change, focusing on spatial strategies, such as ecological networks, short-term refugia, robust corridors, transnational pathways, or potential future protected areas ( (Opdam and Wascher, 2004; ) Thomas, 2005 [NPR] ;( Gaston et al., 2006 ) ). Conservation management for wetlands undergoing erosion has been addressed as well ( Wolters et al., 2005 [ARC] ).
Documented evidence of adaptation to regional climate trends in the highly managed systems of agriculture and forestry is beginning to emerge, such as shifts of sowing dates of annual crops in Europe ( Section 1.3.6 ). With regard to the assessment of vulnerability, few studies have documented observed effects of warming in subsistence agricultural systems in rural populations in developing countries; there are, however, well-documented studies of adaptive responses and vulnerability to long-term drought in the Sahel.
Vulnerability appears to be high in the case of extreme events or exceptional episodes, even in developed countries, as documented by the agricultural response to, and excess mortality occurring in, the 2003 heatwaves in Europe. The global decline in aggregate deaths and death rates due to extreme weather events during the 20th century suggest that adaptation measures to cope with some of the worst consequences of such events have been successful. However, the 2003 European heatwave and the 2005 hurricane season in the North Atlantic show that, despite possessing considerable adaptive capacity, even developed nations are vulnerable if they do not mobilise adaptation measures in a timely and efficient manner. In human health, air-conditioning has contributed to declines in death rates during the summer in the USA and Europe over the past 30-40 years ( Section 1.3.7 ). Documentation of adaptation and vulnerability in terms of energy and tourism is limited ( Section 1.3.9 ).
There is a notable lack of geographical balance in the data and literature on observed changes in natural and managed systems, with a marked scarcity from developing countries. Regions with climate warming with an accumulation of evidence of observed changes in physical and/or biological systems are Europe, Northern Asia, north-western North America, and the Antarctic Peninsula. Regions with warming where evidence of observed changes is sparse are Africa and Latin America, and evidence is lacking in South-east Asia, the Indian Ocean and regions in the Pacific. Possible reasons for this imbalance are lack of access by IPCC authors, lack of data, research and published studies, lack of knowledge of system sensitivity, differing system responses to climate variables, lag effects in responses, resilience in systems and the presence of adaptation. There is a need to improve the observation networks and to enhance research capability on changes in physical, biological and socio-economic systems, particularly in regions with sparse data. This will contribute to an improved functional understanding of the responses of natural and managed systems to climate change.
References
Abeku, T.A., G.J. van Oortmarssen, G. Borsboom, S.J. de Vlas and J.D.F. Habbema, 2003: Spatial and temporal variations of malaria epidemic risk in Ethiopia: factors involved and implications. Acta Trop., 87, 331-340. [SRC]
Abu-Asab, M.S., P.M. Peterson, S.G. Shetler and S.S. Orli, 2001: Earlier plant flowering in spring as a response to global warming in the Washington, DC, area. Biodivers. Conserv., 10, 597. Clean
ACIA, 2005: Arctic Climate Impact Assessment. Cambridge University Press, Cambridge, 1042 pp. [NPR]
Adams, J.B., M.E. Mann and C.M. Ammann, 2003: Proxy evidence for an El Niño-like response to volcanic forcing. Nature, 426, 274-278. [PoC, JoC, MoS]
Adrian, R. and R. Deneke, 1996: Possible impact of mild winters on zooplankton succession in eutrophic lakes of the Atlantic European area. Freshwater Biol., 36, 757-770. Clean
Adrian, R., R. Deneke, U. Mischke, R. Stellmacher and P. Lederer, 1995: A long-term study of the Heiligensee (1975-1992): evidence for effects of climatic change on the dynamics of eutrophied lake ecosystems. Arch. Hydrobiol., 133, 315-337. Clean
Agrawala, S., S. Gigli, V. Raksakulthai, A. Hemp, A. Moehner, D. Conway, M. El Raey, A.U. Ahmed, J. Risbey, W. Baethgen and D. Martino, 2005: Climate change and natural resource management: key themes from case studies. Bridge Over Troubled Waters: Linking Climate Change and Development, S. Agrawala, Ed., Organisation for Economic Co-operation and Development, Paris, 85-131. [NPR, ARC]
Aguiló, E., J. Alegre and M. Sard, 2005: The persistence of the sun and sand tourism model. Tourism Manage., 26, 219-231. [MoS]
Ahas, R., 1999: Long-term phyto-, ornitho- and ichthyophenological time-series analyses in Estonia. Int. J. Biometeorol., 42, 119-123. Clean
Ahas, R., A. Aasa, A. Menzel, V.G. Fedotova and H. Scheifinger, 2002: Changes in European spring phenology. Int. J. Climatol., 22, 1727-1738. [JoC, SRC]
Alexander, M.A. and J.K. Eischeid, 2001: Climate variability in regions of amphibian declines. Conserv. Biol., 15, 930-942. Clean
Allan, J.C. and P.D. Komar, 2006: Climate controls on U.S. West Coast erosion processes. J. Coastal Res., 3, 511-529. Clean
Almaraz, P. and J. Amat, 2004: Complex structural effects of two hemispheric climatic oscillators on the regional spatio-temporal expansion of a threatened bird. Ecol. Lett., 7, 547-556. Clean
Ames, A., 1998: A documentation of glacier tongue variations and lake development in the Cordillera Blanca, Peru. Zeitschrift fur Gletscherkunde and Glazialgeologie, 34, 1-26. Clean
Ames, A., S. Dolores, A. Valverde, P. Evangelista, D. Javier, W. Gavnini, J. Zuniga and V. Gomez, 1989: Glacier Inventory of Peru. Unidad de Glaciología e Hidrología, Huaraz. [NPR]
Amstrup, S.C., 2003: Polar bear, Ursus maritimus. Wild Mammals of North America: Biology, Management, and Conservation, G.A. Feldhamer, B.C. Thompson, J.A. Chapman, Eds., Johns Hopkins University Press, Baltimore, Maryland, 587-610. [NPR]
Amstrup, S.C., I. Stirling, T.S. Smith, C. Perham and G.W. Thiemann, 2006: Recent observations of intraspecific predation and cannibalism among polar bears in the southern Beaufort Sea. Polar Biol., 29, 997-1002. Clean
Andreadis, K.M., E.A. Clark, A.W. Wood, A.F. Hamlet and D.P. Lettenmaier, 2005: Twentieth-century drought in the conterminous United States. J. Hydrometeorol., 6, 985-1001. [JoC, ARC]
Arhonditsis, G.B., M.T. Brett, C.L. DeGasperi and D.E. Schindler, 2004: Effects of climatic variability on the thermal properties of Lake Washington. Limnol. Oceanogr., 49, 256-270. Clean
Arthur, R., T.J. Done and H. Marsh, 2005: Benthic recovery four years after an El Niño-induced coral mass mortality in the Lakshadweep atolls. Curr. Sci. India, 89, 694-699. Clean
Atkinson, A., V. Siegel, E. Pakhomov and P. Rothery, 2004: Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature, 432, 100-103. [JoC]
Attrill, M.J. and M. Power, 2002: Climatic influence on a marine fish assemblage. Nature, 417, 275-278. [JoC]
Bairlein, F. and D.W. Winkel, 2001: Birds and climate change. Climate of the 21st Century: Changes and Risks, J.L. Lozán, H. Graßl and P. Hupfer, Eds., Wissenschaftliche Auswertungen, Hamburg, 278. [NPR]
Barbraud, C. and H. Weimerskirch, 2001: Emperor penguins and climate change. Nature, 411, 183-186. [JoC]
Barnes, K.I., D.N. Durrheim, F. Little, A. Jackson, U. Mehta, E. Allen, S.S. Dlamini, J. Tsoka, B. Bredenkamp, D.J. Mthembu, N.J. White and B.L. Sharp, 2005: Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu–Natal, South Africa. PLoS Med., 2, 1123-1134. Clean
Bartholow, J.M., 2005: Recent water temperature trends in the Lower Klamath River, California. N. Am. J. Fish. Manage., 25, 152-162. Clean
Batimaa, P., 2005: The potential impact of climate change and vulnerability and adaptation assessment for the livestock sector of Mongolia. Assessments of Impacts and Adaptations to Climate Change, AIACC, Washington, District of Columbia, 20 pp. [NPR]
Battarbee, R.W., J.A. Grytnes, R. Thompson, P.G. Appleby, J. Catalan, A. Korhola, H.J.B. Birks, E. Heegaard and A. Lami, 2002: Comparing palaeolimnological and instrumental evidence of climate change for remote mountain lakes over the last 200 years. J. Paleolimnol., 28, 161-179. Clean
Battisti, A., M. Statsmy, A. Schopf, A. Roques, C. Robinet and A. Larsson, 2005: Expansion of geographical range in the pine processionary month caused by increased winter temperature. Ecol. Appl., 15, 2084-2094. Clean
Beare, D., F. Burns, E. Jones, K. Peach, E. Portilla, T. Greig, E. McKenzie and D. Reid, 2004: An increase in the abundance of anchovies and sardines in the north-western North Sea since 1995. Glob. Change Biol., 10, 1209-1213. [JoC]
Beaugrand, G. and P.C. Reid, 2003: Long-term changes in phytoplankton, zooplankton and salmon related to climate. Glob. Change Biol., 9, 801-817. [JoC]
Beaugrand, G., F. Ibanez, J.A. Lindley and P.C. Reid, 2002a: Diversity of calanoid copepods in the North Atlantic and adjacent seas: species associations and biogeography. Mar. Ecol.–Prog. Ser., 232, 179-195. Clean
Beaugrand, G., P.C. Reid, F. Ibanez, J.A. Lindley and M. Edwards, 2002b: Reorganization of North Atlantic marine copepod biodiversity and climate. Science, 296, 1692-1694. [JoC, SRC]
Beaugrand, G., K.M. Brander, J.A. Lindley, S. Souissi and P.C. Reid, 2003: Plankton effect on cod recruitment in the North Sea. Nature, 426, 661-664. [JoC, ARC]
Beaulieu, N. and M. Allard, 2003: The impact of climate change on an emerging coastline affected by discontinuous permafrost: Manitounuk Strait, northern Quebec. Can. J. Earth Sci., 40, 1393-1404. Clean
Beever, E.A., P.E. Brussard and J. Berger, 2003: Patterns of apparent extirpation among isolated populations of pikas (Ochotona princeps) in the Great Basin. J. Mammal., 84, 37-54. Clean
Beggs, P.J., 2004: Impacts of climate change on aeroallergens: past and future. Clin. Exp. Allergy, 34, 1507-1513. [MoS, ARC]
Beggs, P.J. and H.J. Bambrick, 2005: Is the global rise of asthma an early impact of anthropogenic climate change? Environ. Health Persp., 113, 915-919. [ARC]
Bellwood, D.R., A.S. Hoey, J.L. Ackerman and M. Depczynski, 2006: Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Glob. Change Biol., 12, 1587-1594. [JoC]
Beltaos, S., 2002: Effects of climate on mid-winter ice jams. Hydrol. Process., 16, 789-804. Clean
Ben Mohamed, A., N.V. Duivenbooden and S. Abdoussallam, 2002: Impact of climate change on agricultural production in the Sahel. Part 1. Methodological approach and case study for millet in Niger. Climatic Change, 54, 327-348. [JoC]
Beniston, M., 2003: Climatic change in mountain regions: a review of possible impacts. Climatic Change, 59, 5-31. [JoC, ARC]
Beniston, M., 2004: The 2003 heat wave in Europe: a shape of things to come? An analysis based on Swiss climatological data and model simulations. Geophys. Res. Lett., 31, L02202, doi:10.1029/2003GL018857. [JoC, MoS, ARC]
Bennett, L.T. and M.A. Adams, 2004: Assessment of ecological effects due to forest harvesting: approaches and statistical issues. J. Appl. Ecol., 41, 585-598. Clean
Benoit, M. and C.D.L. Torre, 2004: Changement climatique et observation à long terme en unités expérimentales: évolution des pratiques agricoles et des réponses physiologiques des couverts végétaux. Journées MICCES 2004, accessible at: http://www.avignon.inra.fr/les_recherches__1/liste_des_unites/agroclim/mission_changement_climatique_et_effet_de_serre/le_seminaire_de_la_mission_changement_climatique_22_23_janvier_2004/les_posters. [NPR]
Berger, T., J. Mendoza, B. Francou, F. Rojas, R. Fuertes, M. Flores, L. Noriega, C. Ramallo, E. Ramirez and H. Baldivieso, 2005: Glaciares Zongo – Chacaltaya – Charquini Sur – Bolivia 16°S. Mediciones Glaciológicas, Hidrológicas y Meteorológicas, Año Hidrológico 2004-2005. Informe Great Ice Bolivia, IRD-IHH-SENMAHI-COBEE, 171. [NPR, SRC]
Bergeron, Y., M. Flaanigan, S. Gauthier, A. Leduc and P. Lefort, 2004: Past, current and future fire frequency in the Canadian boreal forest: implications for sustainable forest management. Ambio, 33, 356-360. [MoS]
Berkes, F. and D. Jolly, 2002: Adapting to climate change: social-ecological resilience in a Canadian Western Arctic community. Conserv. Ecol., 5, 18. Clean
Berman, M., C. Nicolson, G. Kofinas, J. Tetlichi and S. Martin, 2004: Adaptation and sustainability in a small Arctic community: results of an agent-based simulation model. Arctic, 57, 401-414. [MoS]
Bernatchez, P. and J.-M.M. Dubois, 2004: Bilan des connaissances de la dynamique de l’érosion des côtes du Québec maritime laurentien. Geogr. Phys. Quatern., 58, 45-71. Clean
Berteaux, D., D. Reale, A.G. McAdam and S. Boutin, 2004: Keeping pace with fast climate change: Can arctic life count on evolution? Integr. Comp. Biol., 44, 140. Clean
Berthold, P., E. Gwinner and E. Sonnenschein, 2003: Avian Migration. Springer-Verlag, Berlin, 565 pp. [NPR]
Bertness, M.D., P.J. Ewanchuk and B. Reed, 2002: Anthropogenic modification of New England salt marsh landscapes. P. Natl. Acad. Sci. USA, 99, 1395-1398. [JoC]
Bindoff, N., J. Willebrand, V. Artale, A. Cazenave, J. Gregory, S. Gulev, K. Hanawa, C. Le Quéré, S. Levitus, Y. Nojiri, C.K. Shum, L. Talley and A. Unnikrishnan, 2007: Observations: oceanic climate change and sea level. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller, Eds., Cambridge University Press, Cambridge, 385-432. [NPR, 2007]
Blais, J.M., D.W. Schindler, D.C.G. Muir, M. Sharp, D. Donald, M. Lafreniere, E. Braekevelt and W. M.J. Strachan, 2001: Melting glaciers: a major source of persistent organochlorines to subalpine Bow Lake in Banff National Park, Canada. Ambio, 30, 410-415. Clean
Blaustein, A.R., L.K. Belden, D.H. Olson, D.M. Green, T.L. Root and J.M. Kiesecker, 2001: Amphibian breeding and climate change. Conserv. Biol., 15, 1804. [SRC]
Blenckner, T. and H. Hillebrand, 2002: North Atlantic Oscillation signatures in aquatic and terrestrial ecosystems: a meta-analysis. Glob. Change Biol., 8, 203-212. [JoC]
Bodaly, R.A., J.W.M. Rudd, R.J.P. Fudge and C.A. Kelly, 1993: Mercury concentrations in fish related to size of remote Canadian shield lakes. Can. J. Fish. Aquat. Sci., 50, 980-987. Clean
Boesch, D.F., M.N. Josselyn, A.J. Mehta, J.T. Morris, W.K. Nuttle, C.A. Simenstad and D.J.P. Swift, 1994: Scientific assessment of coastal wetland loss, restoration and management in Louisiana. J. Coast. Res., 20, 1-103. Clean
Boon, S., M. Sharp and P. Nienow, 2003: Impact of an extreme melt event on the runoff and hydrology of a high Arctic glacier. Hydrol. Process., 17, 1051-1072. Clean
Both, C., A.V. Artemyev, B. Blaauw, R.J. Cowie, A.J. Dekhuijzen, T. Eeva, A. Enemar, L. Gustafsson, E.V. Ivankina, A. Jarvinen, N.B. Metcalfe, N.E.I. Nyholm, J. Potti, P.A. Ravussin, J.J. Sanz, B. Silverin, F.M. Slater, L.V. Sokolov, J. Torok, W. Winkel, J. Wright, H. Zang and M.E. Visser, 2004: Large-scale geographical variation confirms that climate change causes birds to lay earlier. P. Roy. Soc. Lond. .B Bio., 271, 1657. Clean
Bowen, N., 2002: Canary in a coalmine. Climbing News, 208, 90-97, 138-139. Clean
Bradshaw, W.E. and C.M. Holzapfel, 2001: Genetic shift in photoperiodic response correlated with global warming. P. Natl. Acad. Sci. USA, 98, 14509. [JoC]
Bradshaw, W.E. and C.M. Holzapfel, 2006: Climate change: evolutionary response to rapid climate change. Science, 312, 1477-1478. [JoC]
Bradshaw, W.E., M.C. Quebodeaux and C.M. Holzapfel, 2003: Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: adaptive response to the photic environment or correlated response to the seasonal environment? Am. Nat., 161, 735. Clean
Brander, K., G. Blom, M.F. Borges, K. Erzini, G. Henderson, B.R. Mackenzie, H. Mendes, J. Ribeiro, A.M.P. Santos and R. Toresen, 2003: Changes in fish distribution in the eastern North Atlantic: are we seeing a coherent response to changing temperature? ICES Marine Science Symposium, 219, 261-270. [ARC]
Briand, J.F., C. Leboulanger, J.F. Humbert, C. Bernard and P. Dufour, 2004: Cylindrospermopsis raciborskii (Cyanobacteria) invasion at mid-latitudes: selection, wide physiological tolerance, or global warming? J. Phycol., 40, 231-238. Clean
Brooks, S.J. and H.J.B. Birks 2004: The dynamics of Chironomidae (Insecta : Diptera) assemblages in response to environmental change during the past 700 years on Svalbard. J. Paleolimnol., 31, 483-498. Clean
Bunn, A.G. and S.J. Goetz, 2006: Trends in satellite-observed circumpolar photosynthetic activity from 1982 to 2003: the influence of seasonality, cover type and vegetation density. Earth Interactions, 10, 1-19. Clean
Burrowes, P.A., R.L. Joglar and D.E. Green, 2004: Potential causes for amphibian declines in Puerto Rico. Herpetologica, 60, 141-154. Clean
Buse, A., S.J. Dury, R.J.W. Woodburn, C.M. Perrins and J.E.G. Good, 1999: Effects of elevated temperature on multi-species interactions: the case of pedunculate oak, winter moth and tits. Funct. Ecol., 13, 74. Clean
Butler, C.J., 2003: The disproportionate effect of global warming on the arrival dates of short-distance migratory birds in North America. Ibis, 145, 484. Clean
Buzin, V.A., A.B. Klaven, Z.D. Kopoliani, V.N. Nikitin and V.I. Teplov, 2004: Results of the studies of the ice jam generation processes and the efficiency of the Lena river hydraulic model at Lensk. Proceedings of the 17th International Symposium on Ice, St. Petersburg, Vol. 3. [NPR]
Camilleri, M., R. Jaques and N. Isaacs, 2001: Impacts of climate change on building performance in New Zealand. Build. Res. Inf., 29, 440-450. Clean
Camuffo, D. and G. Stararo, 2004: Use of proxy-documentary and instrumental data to assess the risk factors leading to sea flooding in Venice. Global Planet. Change, 40, 93-103. Clean
Cannell, M.G.R., J.P. Palutikof and T.H. Sparks, 1999: Indicators of Climate Change in the UK. DETR, London, 87 pp. [NPR, SRC]
Cantelaube, P., J.M. Terres and F.J. Doblas-Reyes, 2004: Climate variability influences on European agriculture: an analysis for winter wheat production. Climate Res., 27, 135-144. Clean
Carey, M., 2005: Living and dying with glaciers: people’s historical vulnerability to avalanches and outburst floods in Peru. Global Planet. Change, 47, 122-134. Clean
Carmichael, W.W., 2001: Health effects of toxin-producing cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk Assess., 7, 1393-1407. Clean
Carminati, E., C. Doglioni and D. Scroccas, 2005. Long term natural subsidence of Venice: evaluation of its causes and magnitude. Flooding and Environmental Challenges for Venice and its Lagoon: State of Knowledge 2003, C. Fletcher and T. Spencer, Eds., Cambridge University Press, Cambridge, 21-28. [NPR]
Carnelli, A.L., J.P. Theurillat, M. Thinon, G. Vadi and B. Talon, 2004: Past uppermost tree limit in the Central European Alps (Switzerland) based on soil and soil charcoal. Holocene, 14, 393-405. Clean
Carson, C., S. Hajat, B. Armstrong and P. Wilkinson, 2006: Declining vulnerability to temperature-related mortality in London over the 20th century. Am. J. Epidemiol., 164, 77-84. Clean
Carvalho, L. and A. Kirika, 2003: Changes in shallow lake functioning: response to climate change and nutrient reduction. Hydrobiologia, 506, 789-796. Clean
Casassa, G. and C. Marangunic, 1993: The 1987 Río Colorado rockslide and debris flow, central Andes, Chile. Bulletin of the Association of Engineering Geologists, 30, 321-330. [SRC]
Casassa, G., A. Rivera, F. Escobar, C. Acuña, J. Carrasco and J. Quintana, 2003: Snow line rise in central Chile in recent decades and its correlation with climate. Joint Assembly. Nice, EAE03-A-14395, CR8-1TH2O-007, EGS-AGU-EUG. [NPR, SRC]
Casman, E., B. Fischhoff, M. Small, H. Dowlatabadi, J. Rose and M.G. Morgan, 2001: Climate change and cryptosporidiosis: a qualitative analysis. Climatic Change, 50, 219-249. [JoC]
Cayan, D.R., S.A. Kammerdiener, M.D. Dettinger, J.M. Caprio and D.H. Peterson, 2001: Changes in the onset of spring in the western United States. B. Am. Meteorol. Soc., 82, 399-415. [JoC, ARC]
Chambers, L.E., L. Hughes and M.A. Weston, 2005: Climate change and its impact on Australia’s avifauna. Emu, 105, 1-20. [ARC]
Charron, D.F., M.K. Thomas, D. Waltner-Toews, J.J. Aramini, T. Edge, R.A. Kent, A.R. Maarouf and J. Wilson, 2004: Vulnerability of waterborne diseases to climate change in Canada: a review. J. Toxicol. Env. Heal. A, 67, 1667-1677. Clean
Chavez, F.P., J. Ryan, S.E. Lluch-Cota and M. Niquen, 2003: From anchovies to sardines and back: multidecadal change in the Pacific Ocean. Science, 299, 217-221. [JoC]
Chelliah, M. and G.D. Bell, 2004: Tropical multidecadal and interannual climate variability in the NCEP-NCAR reanalysis. J. Climate, 17, 1777-1803. [JoC]
Chen, H., X. Chen, B. Hu and R. Yu, 2005a: Spatial and temporal variation of phenological growing season and climate change impacts in temperate eastern China. Glob. Change Biol., 11, 1118-1130. [JoC]
Chen, H., A.K. Githeko, G.F. Zhou, J.I. Githure and G.Y. Yan, 2006: New records of Anopheles arabiensis breeding on the Mount Kenya highlands indicate indigenous malaria transmission. Malaria J., 5, doi:10.1186/1475-2875-5-17. [SRC]
Chen, X., E. Zhang, H. Mu and Y. Zong, 2005b: A preliminary analysis of human impacts on sediment discharges from the Yangtze, China, into the sea. J. Coastal Res., 21, 515-521. Clean
Chen, Z., S.E. Grasby and K.G. Osadetz, 2002: Predicting average annual groundwater levels from climatic variables: an empirical model. J. Hydrol., 260, 102-117. [MoS]
Chiba, S. and K. Tadokoro, 2006: Effects of decadal climate change on zooplankton over the last 50 years in the western subarctic North Pacific. Glob. Change Biol., 12, 907-920. [JoC]
Chmielewski, F.M. and T. Rotzer, 2001: Response of tree phenology to climate change across Europe. Agr. Forest Meteorol., 108, 101-112. Clean
Chmielewski, F.M., A. Muller and E. Bruns, 2004: Climate changes and trends in phenology of fruit trees and field crops in Germany, 1961-2000. Agr. Forest Meteorol., 121, 69-78. Clean
Church, J.A. and N.J. White, 2006: A 20th century acceleration in global sea-level rise. Geophys. Res. Lett., 33, L01602, doi:10.1029/2005GL024826. [JoC]
Church, J.A., N.J. White, R. Coleman, K. Lambeck and J.X. Mitrovica, 2004: Estimates of the regional distribution of sea-level rise over the 1950-2000 period. J. Climate, 17, 2609-2625. [JoC, MoS]
Clark, D.A., S.C. Piper, C.D. Keeling and D.B. Clark, 2003: Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984-2000. P. Natl. Acad. Sci. USA, 100, 5852-5857. [JoC]
Community of Arctic Bay, S. Nickels, C. Furgal, J. Akumilik and B.J. Barnes, 2005: Unikkaaqatigiit: Putting the Human Face on Climate Change – Perspectives from Arctic Bay, Nunavut. Report of the Workshop held in Arctic Bay, Nunavut, March 3–4, 2004. Joint publication of Inuit Tapiriit Kanatimi, Nasivvik Centre for Inuit Health and Changing Environments at Université Laval and the Ajunnginiq Centre at the National Aboriginal Health Organization, Ottawa, 31 pp, http://www.itk.ca/environment/climate-book/community/ArcticBay.pdf. [NPR, ARC]
Conard, S.G., A.I. Sukhinin, B.J. Stocks, D.R. Cahoon, E.P. Davidenko and G.A. Ivanova, 2002: Determining effects of area burned and fire severity on carbon cycling and emissions in Siberia. Climatic Change, 55, 197-211. [JoC]
Cooke, S.J., S.G. Hinch, A.P. Farrell, M.F. Lapointe, S.R.M. Jones, J.S. Macdonald, D.A. Patterson, M.C. Healey and G. Van Der Kraak, 2004: Abnormal migration timing and high en route mortality of sockeye salmon in the Fraser River, British Columbia. Fisheries, 29, 22-33. Clean
Cooper, N.J., T. Cooper and F. Burd, 2001: 25 years of salt marsh erosion in Essex: implications from coastal defence and natural conservation. Journal of Coastal Conservation, 9, 31-40. Clean
Cotton, P.A., 2003: Avian migration phenology and global climate change. P. Natl. Acad. Sci. USA, 100, 12219-12222. [JoC]
Coudrain, A., B. Francou and Z.W. Kundzewicz, 2005: Glacier shrinkage in the Andes and consequences for water resources: editorial. Hydrolog. Sci. J., 50, 925–932. [SRC]
Couture, R., S.D. Robinson and M.M. Burgess, 2000: Climate change, permafrost, degradation and infrastructure adaptation: preliminary results from a pilot community study in the Mackenzie Valley. Geological Survey of Canada Current Research, 2000, 1-9. Clean
Craig, M.H., I. Kleinschmidt, D. Le Sueur and B.L. Sharp, 2004: Exploring thirty years of malaria case data in KwaZulu-Natal, South Africa. Part II. The impact of non-climatic factors. Trop. Med. Int. Health, 9, 1258-1266. Clean
Crick, H.Q.P. and T.H. Sparks, 1999: Climate change related to egg-laying trends. Nature, 399, 423-424. [JoC, SRC]
Crick, H.Q.P., C. Dudley, D.E. Glue and D.L. Thomson, 1997: UK birds are laying eggs earlier. Nature, 388, 526. [JoC]
Crozier, L., 2004: Warmer winters drive butterfly range expansion by increasing survivorship. Ecology, 85, 231-241. Clean
Cullen, L.E., G.H. Stewart, R.P. Duncan and J.G. Palmer, 2001: Disturbance and climate warming influences on New Zealand Nothofagus tree-line population dynamics. J. Ecol., 89, 1061. Clean
Curriero, F.C., J.A. Patz, J.B. Rose and S. Lele, 2001: The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948-1994. Am. J. Public Health, 91, 1194-1199. [ARC]
Curriero, F., K.S. Heiner, J. Samet, S. Zeger, L. Strug and J.A. Patz, 2002: Temperature and mortality in 11 cities of the Eastern United States. Am. J. Epidemiol., 155, 80-87. [ARC]
Dabrowski, M., W. Marszelewski and R. Skowron, 2004: The trends and dependencies between air and water temperatures in lakes in northern Poland in 1961-2000. Hydrol. Earth Syst. Sc., 8, 79-87. Clean
Dai, A., K.E. Trenberth and T. Qian, 2004: A global data set of Palmer Drought Severity Index for 1870-2002: relationship with soil moisture and effect of surface warming. J. Hydrometeorol., 5, 1117-1130. [PoC, JoC]
Daniel, H., 2001: Replenishment versus retreat: the cost of maintaining. Ocean Coast. Manage., 44, 87-104. [JoC]
Daniel, M., V. Danielová, B. K?í?, A. Jirsa and J. No?i?ka, 2003: Shift of the tick Ixodes ricinus and tick-borne encephalitis to higher altitudes in central Europe. Eur. J. Clin. Microbiol., 22, 327-328. Clean
Danielová, V., N. Rudenko, M. Daniel, J. Holubova, J. Materna, M. Golovchenko and L. Schwarzova, 2006: Extension of Ixodes ricinus ticks and agents of tick-borne diseases to mountain areas in the Czech Republic. Int. J. Med. Microbiol., 296, 48-53. Clean
D’Arrigo, R.D., R.K. Kaufmann, N. Davi, G.C. Jacoby, C. Laskowski, R.B. Myneni and P. Cherubini, 2004: Thresholds for warming-induced growth decline at elevational tree line in the Yukon Territory, Canada. Global Biogeochem. Cy., 18, GB3021. Clean
Daufresne, M., M.C. Roger, H. Capra and N. Lamouroux, 2004: Long-term changes within the invertebrate and fish communities of the Upper Rhone River: effects of climatic factors. Glob. Change Biol., 10, 124-140. [JoC]
Davies, Z.G., R.J. Wilson, S. Coles and C.D. Thomas, 2006: Changing habitat associations of a thermally constrained species, the silver-spotted skipper butterfly, in response to climate warming. J. Anim. Ecol., 75, 247-256. Clean
Davis, M.A., J.P. Grime and K. Thompson, 2000: Fluctuating resources in plant communities: a general theory of invasibility. J. Ecol., 88, 528-534. Clean
Davis, R.E., P.C. Knappenberger, P.J. Michaels and W.M. Novicoff, 2003a: Changing heat-related mortality in the United States. Environ. Health Persp., 111, 1712-1718. Clean
Davis, R.E., P.C. Knappenberger, W.M. Novicoff and P.J. Michaels, 2003b: Decadal changes in summer mortality in US cities. Int. J. Biometeorol., 47, 166-175. Clean
Day, J.W., G. Abrami, J. Rybczyk and W. Mitsch, 2005. Venice Lagoon and the Po Delta: system functioning as a basis for sustainable management. Flooding and Environmental Challenges for Venice and its Lagoon: State of Knowledge, C.A. Fletcher and T. Spencer, Eds., Cambridge University Press, Cambridge, 445-459. [NPR]
de Dear, R.J. and G.S. Brager, 1998: Developing an adaptive model of thermal comfort and preference. ASHRAE Transactions, 104, 145-167. [MoS]
Defila, C. and B. Clot, 2001: Phytophenological trends in Switzerland. Int. J.Biometeorol., 45, 203-207. [ARC]
Defra, 2003: The Impacts of Climate Change: Implications for Defra. Department for Environment, Food and Rural Affairs, 40 pp, http://www.defra.gov.uk/ENVIRONMENT/climatechange/pubs/impacts/pdf/ccimpacts_defra.pdf. [NPR]
Delbart, N., T. Le Toan, L. Kergoat and V. Fedotova, 2006: Remote sensing of spring phenology in boreal regions: a free of snow-effect method using NOAA-AVHRR and SPOT-VGT data (1982-2004). Remote Sens. Environ., 101, 52-62. Clean
Derocher, A.E., N.J. Lunn and I. Stirling, 2004: Polar bears in a warming climate. Integr. Comp. Biol., 44, 163-176. [ARC]
Dery, S.J. and E.F. Wood, 2005: Decreasing river discharge in northern Canada. Geophys. Res. Lett., 32, L10401, doi: 10.1029/2005GL022845. [JoC]
Diaz, J., A. Jordan, R. Garcia, C. Lopez, J.C. Alberdi, E. Hernandez and A. Otero, 2002: Heat waves in Madrid 1986-1997: effects on the health of the elderly. Int. Arch. Occ. Env. Hea., 75, 163-170. Clean
Diergaardt, S.M., S.N. Venter, A. Spreeth, J. Theron and V.S. Brozel, 2004: The occurrence of campylobacters in water sources in South Africa. Water Res., 38, 2589-2595. Clean
Dobbertin, M., N. Hilker, M. Rebetez, N.E. Zimmermann, T. Wohlgemuth and A. Rigling, 2005: The upward shift in altitude of pine mistletoe (Viscum album ssp. austriacum) in Switzerland: the result of climate warming? Int. J. Biometeorol., 50, 40-47. Clean
Doledec, S., J. Dessaix and H. Tachet, 1996: Changes within the Upper Rhone River macrobenthic communities after the completion of three hydroelectric schemes: anthropogenic effects or natural change? Arch. Hydrobiol., 136, 19-40. Clean
Donaldson, G.C., W.R. Keatinge and S. Näyhä, 2003: Changes in summer temperature and heat-related mortality since 1971 in North Carolina, South Finland and Southeast England. Environ. Res., 91, 1-7. Clean
Donnelly, A., M.B. Jones and J. Sweeney, 2004: A review of indicators of climate change for use in Ireland. Int. J. Biometeorol., 49, 1-12. [ARC]
Donnelly, J.P. and M.D. Bertness, 2001: Rapid shoreward encroachment of salt marsh cordgrass in response to accelerated sea-level rise. P. Natl. Acad. Sci. USA, 98, 14218-14223. [JoC]
Dose, V. and A. Menzel, 2004: Bayesian analysis of climate change impacts in phenology. Glob. Change Biol., 10, 259-272. [JoC, SRC]
Dose, V. and A. Menzel, 2006: Bayesian correlation between temperature and blossom onset data. Glob. Change Biol., 12, 1451-1459. [JoC, SRC]
Douglas, E.M., R.M. Vogel and C.N. Kroll, 2000: Trends in floods and low flows in the United States: impacts of spatial correlation. J. Hydrol., 240, 90-105. Clean
Douglas, M.S.V., J.P. Smol and W. Blake, 1994: Marked post-18th century environmental-change in high-Arctic ecosystems. Science, 266, 416-419. [JoC]
Drinkwater, K.F., A. Belgrano, A. Borja, A. Conversi, M. Edwards, C.H. Greene, G. Ottersen, A.J. Pershing and H. Walker, 2003: The response of marine ecosystems to North Atlantic climate variability associated with the North Atlantic Oscillation. The North Atlantic Oscillation: Climate Significance and Environmental Impact, J. Hurrell, Y. Kushnir, G. Ottersen and M. Visbeck, Eds., Geophysical Monograph 134, American Geophysical Union, Washington, District of Columbia, 211-234. [NPR, SRC]
D’Souza, R.M., N.G. Beeker, G. Hall and K.B.A. Moodie, 2004: Does ambient temperature affect foodborne disease? Epidemiology, 15, 86-92. Clean
Du, M.Y., S. Kawashima, S. Yonemura, X.Z. Zhang and S.B. Chen, 2004: Mutual influence between human activities and climate change in the Tibetan Plateau during recent years. Global Planet. Change, 41, 241-249. Clean
Duchêne, E. and C. Schneider, 2005: Grapevine and climatic changes: a glance at the situation in Alsace. Agron. Sustain. Dev., 25, 93-99. Clean
Dul?i?, J., B. Grbec, L. Lipej, G. Beg-Paklar, N. Supic and A. Smircic, 2004: The effect of the hemispheric climatic oscillations on the Adriatic ichthyofauna. Fresen. Environ. Bull., 13, 293-298. Clean
Dunn, P., 2004. Breeding dates and reproductive performance. Adv. Ecol. Res., 35, 69-87. Clean
Dunn, P.O. and D.W. Winkler, 1999: Climate change has affected the breeding date of tree swallows throughout North America. P. Roy. Soc. Lond. B Bio., 266, 2487. Clean
Eagles, P.F.J., 2004: Trends affecting tourism in protected areas. Policies, Methods and Tools for Visitor Management: Proceedings of the Second International Conference on Monitoring and Management of Visitor Flows in Recreational and Protected Areas, Rovaniemi, Finland, June 16–20, 2004, T. Sievänen, J. Erkkonen, J. Jokimäki, J. Saarinen, S. Tuulentie and E. Virtanen, Eds., Working Papers of the Finnish Forest Research Institute 2, 17-25. [NPR]
Eakin, H., 2000: Smallholder maize production and climate risk: a case study from Mexico. Climatic Change, 45, 19-36. [JoC]
Easterling, D.R., G.A. Meehl, C. Parmesan, S.A. Changnon, T.R. Karl and L.O. Mearns, 2000: Climate extremes: observations, modeling, and impacts. Science, 289, 2068-2074. [PoC, JoC, MoS, ARC]
Easterling, W.E., 2003: Observed impact of climate change in agriculture and forestry. IPCC Workshop on the Detection and Attribution of the Effects of Climate Change, GISS, New York, 54-55. [NPR, ARC]
Edwards, M. and A.J. Richardson, 2004: Impact of climate change on marine pelagic phenology and trophic mismatch. Nature, 430, 881-884. [JoC, SRC]
Edwards, M., P. Reid and B. Planque, 2001: Long-term and regional variability of phytoplankton biomass in the Northeast Atlantic (1960-1995). ICES J. Mar. Sci., 58, 39-49. [SRC]
Edwards, M., G. Beaugrand, P.C. Reid, A.A. Rowden and M.B. Jones, 2002: Ocean climate anomalies and the ecology of the North Sea. Mar. Ecol.–Prog. Ser., 239, 1-10. [SRC]
Edwards, M., D.G. Johns, S.C. Leterme, E. Svendsen and A.J. Richardson, 2006: Regional climate change and harmful algal blooms in the northeast Atlantic. Limnol. Oceanogr., 51, 820-829. [SRC]
EEA, 2004: Impacts of Europe’s Changing Climate: An Indicator-based Assessment. European Environment Agency, Copenhagen, Report 2/2004. Office for Official Publications of the European Communities, Luxembourg, 107 pp. [NPR]
Elliott, J.M., M.A. Hurley and S.C. Maberly, 2000: The emergence period of sea trout fry in a Lake District stream correlates with the North Atlantic Oscillation. J. Fish Biol., 56, 208-210. Clean
Elsasser, H. and P. Messerli, 2001: The vulnerability of the snow industry in the Swiss Alps. Mt. Res. Dev., 21, 335-339. Clean
Emanuel, K., 2005: Increasing destructiveness of tropical cyclones over the past 30 years. Nature, 434, 686-688. [JoC]
Emberlin, J., M. Detandt, R. Gehrig, S. Jaeger, N. Nolard and A. Rantio-Lehtimaki, 2003: Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperatures across Europe. Int. J. Biometeorol., 47, 113-115. Clean
Erdenetuya, M., 2004: Application of remote sensing in climate change study: vulnerability and adaptation assessment for grassland ecosystem and livestock sector in Mongolia project. AIACC Annual Report, Washington, DC. [NPR]
Ericson, J.P., C.J. Vorosmarty, S.L. Dingman, L.G. Ward and M. Meybeck, 2006: Effective sea-level rise and deltas: causes of change and human dimension implications. Global Planet. Change, 50, 63-82. Clean
Estrella, N. and A. Menzel, 2006: Responses of leaf colouring in four deciduous tree species to climate and weather in Germany. Climate Res., 32, 253-267. [SRC]
Estrella, N., T.H. Sparks and A. Menzel, 2007: Trends and temperature response in the phenology of crops in Germany. Glob. Change Biol., doi:10.1111/j.1365-2486.2007.01374.x. [NPR, SRC, 2007]
Fang, S. and K. Dingbo, 2003: Remote sensing investigation and survey of Qinghai Lake in the past 25 years. Journal of Lake Sciences, 15, 290-296. Clean
Feely, R.A., C.L. Sabine, K. Lee, W. Berelson, J. Kleypas, V.J. Fabry and F.J. Millero, 2004: Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science, 305, 362-366. [JoC]
Feng, S. and Q. Hu, 2004: Changes in agro-meteorological indicators in the contiguous United States: 1951-2000. Theor. Appl. Climatol., 78, 247-264. Clean
Ferguson, G. and S.S. George, 2003: Historical and estimated ground water levels near Winnipeg, Canada and their sensitivity to climatic variability. J. Am. Water Resour. As., 39, 1249-1259. [MoS]
Field, D.B., T.R. Baumgarter, C.D. Charles, V. Ferreira-Bartrina and M.D. Ohman, 2006: Planktonic foraminifera of the California Current reflect 20th-century warming. Science, 311, 63-66. [JoC]
Fillol, E.J. and A. Royer, 2003: Variability analysis of the transitory climate regime as defined by the NDVI/Ts relationship derived from NOAA-AVHRR over Canada. IEEE Int., 4, 21-25. Clean
Fitter, A.H. and R.S.R. Fitter, 2002: Rapid changes in flowering time in British plants. Science, 296, 1689. [JoC]
Forbes, D.L., 2005: Coastal erosion. Encyclopedia of the Arctic, M. Nuttall, Ed., Routledge, New York and London, 391-393. [NPR, SRC]
Forbes, D.L., G.S. Parkes, G.K. Manson and L.A. Ketch, 2004: Storms and shoreline retreat in the southern Gulf of St. Lawrence. Mar. Geol., 210, 169-204. [SRC]
Forcada, J., P.N. Trathan, K. Reid and E.J. Murphy, 2005: The effects of global climate variability in pup production on Antarctic fur seals. Ecol. Lett., 86, 2408-2417. Clean
Forcada, J., P.N. Trathan, K. Reid, E.J. Murphy and J.P. Croxall, 2006: Contrasting population changes in sympatric penguin species in association with climate warming. Glob. Change Biol., 12, 411-423. [JoC]
Ford, J.D. and B. Smit, 2004: A framework for assessing the vulnerability of communities in the Canadian Arctic to risks associated with climate change. Arctic, 57, 389-400. [ARC]
Forister, M.L. and A.M. Shapiro, 2003: Climatic trends and advancing spring flight of butterflies in lowland California. Glob. Change Biol., 9, 1130-1135. [JoC]
Fox, S., 2002: These things that are really happening: Inuit perspectives on the evidence and impacts of climate change in Nunavut. The Earth is Faster Now: Indigenous Observations of Arctic Environmental Change, I. Krupnik and D. Jolly, Eds., Arctic Research Consortium of the US, Fairbanks, Alaska, 12-53. [NPR]
Franco, A.M.A., J.K. Hill, C. Kitschke, Y.C. Collingham, D.B. Roy, R. Fox, B. Huntley and C.D. Thomas, 2006: Impacts of climate warming and habitat loss on extinctions at species’ low-latitude range boundaries. Glob. Change Biol., 12, 1545-1553. [JoC]
Francou, B. and C. Vincent, 2006: Les glaciers à l’épreuve du climat. IRD/BELIN, Paris, 274 pp. [NPR, SRC]
Francou, B., M. Vuille, P. Wagnon, J. Mendoza and J.E. Sicart, 2003: Tropical climate change recorded by a glacier of the central Andes during the last decades of the 20th century: Chacaltaya, Bolivia, 16°S. J. Geophys. Res., 108, 4154. [JoC, SRC]
Francou, B., P. Ribstein, P. Wagnon, E. Ramirez and B. Pouyaud, 2005: Glaciers of the tropical Andes: indicators of the global climate variability. Global Change and Mountain Regions: A State of Knowledge Overview, U.M. Huber, H.K.M. Bugmann and M.A. Reasoner, Eds., Advances in Global Change Research, Vol 23, Springer, Berlin, 197-204. [NPR, SRC]
Franken, R.J. and D.S. Hik, 2004: Interannual variation in timing of parturition and growth of collared pikas (Ochotona collaris) in the southwest Yukon. Integr. Comp. Biol., 44, 186. Clean
Frederiksen, M., M.P. Harris, F. Daunt, P. Rothery and S. Wanless, 2004: Scale-dependent climate signals drive breeding phenology of three seabird species. Glob. Change Biol., 10, 1214-1221. [JoC]
Frei, C. and C. Schar, 2001: Detection probability of trends in rare events: theory and application to heavy precipitation in the Alpine region. J. Climate, 14, 1568-1584. [JoC]
Frenot, Y., S.L. Chown, J. Whinam, P.M. Selkirk, P. Convey, M. Skotnicki and D.M. Bergstrom, 2005: Biological invasions in the Antarctic: extent, impacts and implications. Biol. Rev., 80, 45-72. Clean
Frihy, O., K. Dewidar and M. El Raey, 1996: Evaluation of coastal problems at Alexandria, Egypt. Ocean Coast. Manage., 30, 281-295. [JoC]
Frolov, A.V., S.V. Borshch, E.S. Dmitriev, M.V. Bolgov and N.I. Alekseevsky, 2005: Dangerous hydrological events: methods for analysis and forecasting, mitigation of negative results. Proceedings of the VIth All-Russia Hydrological Congress, Vol.1, St .Petersburg. [NPR]
Fromentin, J.M. and B. Planque, 1996: Calanus and environment in the eastern North Atlantic. II. Influence of the North Atlantic Oscillation on Calanus finmarchicus and C. hegolandicus. Mar. Ecol.–Prog. Ser., 134, 111-118. [NPR]
Fruget, J.F., M. Centofanti, J. Dessaix, J.M. Olivier, J.C. Druart and P.J. Martinez, 2001: Temporal and spatial dynamics in large rivers: example of a long-term monitoring of the Middle Rhone River. Ann. Limnol.–Int. J. Lim., 37, 237-251. Clean
Gajewski, K., P.B. Hamilton and R. McNeely, 1997: A high resolution proxy-climate record from an arctic lake with annually-laminated sediments on Devon Island, Nunavut, Canada. J. Paleolimnol., 17, 215-225. Clean
Gao, Y., G.Y. Qiu, H. Shimizu, K. Tobe, B.P. Sun and J. Wang, 2002: A 10-year study on techniques for vegetation restoration in a desertified salt lake area. J. Arid Environ., 52, 483-497. Clean
Gardner, T.A., I.M. Côté, J.A. Gill, A. Grant and A.R. Watkinson, 2005: Hurricanes and Caribbean coral reefs: impacts, recovery patterns and role in long-term decline. Ecology, 86, 174-184. [SRC]
Garpe, K.C., S.A.S. Yahya, U. Lindahl and M.C. Ohman, 2006: Long-term effects of the 1998 coral bleaching event on reef fish assemblages. Mar. Ecol.–Prog. Ser., 315, 237-247. Clean
Gaston, K.J., K. Charman, S.F. Jackson, P.R. Armsworth, A. Bonn, R.A. Briers, C.S.Q. Callaghan, R. Catchpole, J. Hopkins, W.E. Kunin, J. Latham, P. Opdam, R. Stoneman, D.A. Stroud and R. Tratt, 2006: The ecological effectiveness of protected areas: the United Kingdom. Biol. Conserv., 132, 76-87. Clean
Gedney, N., P.M. Cox, R.A. Betts, O. Boucher, C. Huntingford and P.A. Stott, 2006: Detection of a direct carbon dioxide effect in continental river runoff records. Nature, 439, 835-838. [JoC, ARC]
Genner, M.J., D.W. Sims, V.J. Wearmouth, E.J. Southall, A.J. Southward, P.A. Henderson and S.J. Hawkins, 2004: Regional climatic warming drives long-term community changes of British marine fish. P. Roy. Soc. Lond. B Bio., 271, 655-661. Clean
Genovese, G., C. Lazar and F. Micale, 2005: Effects of observed climate fluctuation on wheat flowering as simulated by the European crop growth monitoring system (CGMS). Proceedings of a Workshop on Adaptation of Crops and Cropping Systems to Climate Change, 7-8 November 2005, Dalum Landbrugsskole, Odense, Denmark. Nordic Association of Agricultural Scientists, 12 pp. [NPR, MoS]
Georgiou, I.Y., D.M. FitzGerald and G.W. Stone, 2005: The impact of physical processes along the Louisiana coast. J. Coastal Res., 44, 72-89. Clean
Gerten, D. and R. Adrian, 2000: Climate-driven changes in spring plankton dynamics and the sensitivity of shallow polymictic lakes to the North Atlantic Oscillation. Limnol. Oceanogr., 45, 1058-1066. Clean
Gerten, D. and R. Adrian, 2002: Species-specific changes in the phenology and peak abundance of freshwater copepods in response to warm summers. Freshwater Biol., 47, 2163-2173. Clean
Gibbs, J.P. and A.R. Breisch, 2001: Climate warming and calling phenology of frogs near Ithaca, New York, 1900-1999. Conserv. Biol., 15, 1175. Clean
Gill, J.A., J.P. McWilliams, A.R. Watkinson and I.M. Côté, 2006: Opposing forces of aerosol cooling and El Niño drive coral bleaching on Caribbean reefs. P. Natl. Acad. Sci. USA, 103, 18870-18873. [JoC, SRC]
Gillett, N.P., A.J. Weaver, F.W. Zwiers and M.D. Flannigan, 2004: Detecting the effect of climate change on Canadian forest fires. Geophys. Res. Lett., 31, L18211, doi:10.1029/2004GL020876. [JoC]
Githeko, A. and W. Ndegwa, 2001: Predicting malaria epidemics in the Kenya highlands using climate data: a tool for decision makers. Glob. Change Hum. Health, 2, 54-63. [MoS, SRC]
Githeko, A. and A. Woodward, 2003: International consensus on the science of climate and health: the IPCC Third Assessment Report. Climate Change and Human Health: Risks and Responses, A.J. McMicheal, D.H. Campbell-Lendrum, C.F. Corvalan, K.L. Ebi, A. Githeko, J.D. Scheraga and A. Woodward, Eds., World Health Organization, Rome, 43-60. www.who.int/globalchange/publications/climatechangechap3.pdf. [NPR, SRC]
Gobron, N., B. Pinty, F. Melin, M. Taberner, M.M. Verstraete, A. Belward, T. Lavergne and J.L. Widlowski, 2005: The state of vegetation in Europe following the 2003 drought. Int. J. Remote Sens., 26, 2013-2020. Clean
Goetz, S.J., A.G. Bunn, G.J. Fiske and R.A. Houghton, 2005: Satellite-observed photosynthetic trends across boreal North America associated with climate and fire disturbance. P. Natl. Acad. Sci. USA, 102, 13521-13525. [JoC]
Goklany, I.M., 1996: Factors affecting environmental impacts: the effects of technology on long-term trends in cropland, air pollution and water-related diseases. Ambio, 25, 497-503. Clean
Goldenberg, S.B., C.W. Landsea, A.M. Mestas-Nunez and W.M. Gray, 2001: The recent increase in Atlantic hurricane activity: causes and implications. Science, 293, 474-479. [JoC]
Gonzalez, P., 2001: Desertification and a shift of forest species in the West African Sahel. Climate Res., 17, 217-228. Clean
Grabherr, G., M. Gottfried and H. Pauli, 2001: Long-term monitoring of mountain peaks in the Alps. Biomonitoring: General and Applied Aspects on Regional and Global Scales – Tasks for Vegetation Science, C.A. Burga and A. Kratochwil, Eds., Kluwer Academic, Dordrecht, 153- 177. [NPR]
Graham, N.A.J., S.K. Wilson, S. Jennings, N.V.C. Polunin, J.P. Bijoux and J. Robinson, 2006: Dynamic fragility of oceanic coral reef ecosystems. P. Natl. Acad. Sci. USA, 103, 8425-8429. [JoC, ARC]
Grebmeier, J.M., J.E. Overland, S.E. Moore, E.V. Farley, E.C. Carmack, L.W. Cooper, K.E. Frey, J.H. Helle, F.A. McLaughlin and S.L. McNutt, 2006: A major ecosystem shift in the northern Bering Sea. Science, 311, 1461-1464. [JoC]
Green, K. and C.M. Pickering, 2002: A potential scenario for mammal and bird diversity in the Snowy Mountains of Australia in relation to climate change. Mountain Biodiversity: A Global Assessment, C. Korner and E. Spehn, Eds., Parthenon Publishing, London, 241-249. [NPR]
Gregg, W.W., M.E. Conkright, P. Ginoux, J.E. O’Reilly and N.W. Casey, 2003: Ocean primary production and climate: global decadal changes. Geophys. Res. Lett., 30, 1809, doi:10.1029/2003GL016889. [JoC]
Grenouillet, G., B. Hugueny, G.A. Carrel, J.M. Olivier and D. Pont, 2001: Large-scale synchrony and inter-annual variability in roach recruitment in the Rhone River: the relative role of climatic factors and density-dependent processes. Freshwater Biol., 46, 11-26. Clean
Greve, W., 2004: Aquatic plants and animals. Phenology: An Integrative Environmental Science, M. D. Schwartz, Ed., Kluwer, Dordrecht, 385-403. [NPR]
Gruber, S., M. Hoelzle and W. Haeberli, 2004: Permafrost thaw and destabilization of Alpine rock walls in the hot summer of 2003. Geophys. Res. Lett., 31, L13504, doi:10.1029/2004GL020051. [JoC, SRC]
Guisande, C., A. Vergara, I. Riveiro and J. Cabanadas, 2004: Climate change and abundance of the Atlantic-Iberian sardine (Sardina pilchardus). Fish. Oceanogr., 13, 91-101. Clean
Günther, H., W. Rosenthal, M. Stawarz, J.C. Carretero, M. Gomez, I. Lozano, O. Serano and M. Reistad, 1998: The wave climate of the Northeast Atlantic over the period 1955-94: the WASA wave hindcast. The Global Atmosphere and Ocean System, 6, 121-163. Clean
Gyan, K., W. Henry, S. Lacaille, A. Laloo, C. Lamsee-Ebanks, S. McKay, R. Antoine and M. Monteil, 2003: African dust clouds are associated with paediatric accident and emergency asthma admissions at the Eric Williams Medical Sciences Complex. W. Indian Med. J., 52, 46. Clean
Haeberli, W. and C. Burn, 2002: Natural hazards in forests - glacier and permafrost effects as related to climate changes. Environmental Change and Geomorphic Hazards in Forests, R.C. Sidle, Ed., IUFRO Research Series, 9, 167-202. [NPR, SRC]
Haeberli, W., A. Kääb, D. Vonder Mühll, and P. Teysseire, 2001: Prevention of outburst floods from periglacial lakes at Grubengletscher, Valais, Swiss Alps. J. Glaciol., 47, 111-122. [SRC]
Hafner, S., 2003: Trends in maize, rice and wheat yields for 188 nations over the past 40 years: a prevalence of linear growth. Agr. Ecosyst. Environ., 97, 275-283. Clean
Hambright, K.D., M. Gophen and S. Serruya, 1994: Influence of long-term climatic changes on the stratification of a subtropical, warm monomictic lake. Limnol. Oceanogr., 39, 1233-1242. Clean
Hamilton, J.M., 2003a: Climate and the destination choice of German tourists, Working Paper FNU-15 (revised), Centre for Marine and Climate Research, University of Hamburg, 36 pp. [NPR]
Hamilton, J.M., D.J. Maddison and R.S.J. Tol, 2003: Climate change and international tourism: a simulation study. Working Paper FNU-31, Research Unit Sustainability and Global Change, 41 pp. [NPR, MoS, ARC]
Hamilton, J.M., D.J. Maddison and R.S.J. Tol, 2005: Climate change and international tourism: a simulation study. Global Environ. Chang., 15, 253-266. [JoC, MoS, ARC]
Hamilton, L.C., 2003b: Warming winters and New Hampshire’s lost ski areas: an integrated case study. Int. J. Sociol. Social Pol., 23, 52-73. Clean
Hampton, S.E., 2005: Increased niche differentiation between two Conochilus species over 33 years of climate change and food web alteration. Limnol. Oceanogr., 50, 421-426. Clean
Hari, R.E., D.M. Livingstone, R. Siber, P. Burkhardt-Holm and H. Guttinger, 2006: Consequences of climatic change for water temperature and brown trout populations in Alpine rivers and streams. Glob. Change Biol., 12, 10-26. [JoC]
Hartig, E.K. and V. Gornitz, 2004: Salt marsh change, 1926-2002 at Marshlands Conservancy, New York. Proceedings of the Long Island Sound Biennial Research Conference, 7 pp. [NPR, SRC]
Hartig, E.K., V. Gornitz, A. Kolker, F. Mushacke and D. Fallon, 2002: Anthropogenic and climate-change impacts on salt marshes of Jamaica Bay, New York City. Wetlands, 22, 71-89. [SRC]
Harvell, C.D., K. Kim, J.M. Burkholder, R.R. Colwell, P.R. Epstein, D.J. Grimes, E.E. Hofmann, E.K. Lipp, A.D.M.E. Osterhaus, R.M. Overstreet, J.W. Porter, G.W. Smith and G.R. Vasta, 1999: Emerging marine diseases: climate links and anthropogenic factors. Science, 285, 1505-1510. [JoC]
Haslett, S.K., A.B. Cundy, C.F.C. Davies, E.S. Powell and I.W. Croudace, 2003: Salt marsh, sedimentation over the past c. 120 years along the West Cotentin coast of Normandy (France): relationship to sea-level rise and sediment supply. J. Coastal Res., 19, 609-620. Clean
Hattenschwiler, S. and C. Korner, 2003: Does elevated CO2 facilitate naturalization of the non-indigenous Prunus laurocerasus in Swiss temperate forests? Funct. Ecol., 17, 778. [ARC]
Hawkins, S.J., A.J. Southward and M.J. Genner, 2003: Detection of environmental change in a marine ecosystem: evidence from the western English Channel. Sci. Total Environ., 310, 245-256. Clean
Hay, S.I., J. Cox, D.J. Rogers, S.E. Randolph, D.I. Stern, G.D. Shanks, M.F. Myers and R.W. Snow, 2002a: Climate change and the resurgence of malaria in the East African highlands. Nature, 415, 905-909. [JoC]
Hay, S.I., D.J. Rogers, S.E. Randolph, D.I. Stern, J. Cox, G.D. Shanks and R.W. Snow, 2002b: Hot topic or hot air? Climate change and malaria resurgence in East African highlands. Trends Parasitol., 18, 530-534. Clean
Hecky, R.E., F.W.B. Bugenyi, P. Ochumba, J.F. Talling, R. Mugidde, M. Gophen and L. Kaufman, 1994: Deoxygenation of the deep-water of Lake Victoria, East Africa. Limnol. Oceanogr., 39, 1476-1481. Clean
Hegerl, G.C., F.W. Zwiers, P. Braconnot, N.P. Gillett, Y. Luo, J. Marengo, N. Nicholls, J.E. Penner and P.A. Stott, 2007: Understanding and attributing climate change. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller, Eds., Cambridge University Press, Cambridge, 663-746. [NPR, ARC, 2007]
Helander, E. and T. Mustonen, Eds., 2004: Snowscapes, Dreamscapes: Snowchange Book on Community Voices of Change. Tampere Polytechnic Publications, Oy, Vaasa, 562 pp. [NPR]
Hickling, R., D.B. Roy, J.K. Hill and C.D. Thomas, 2005: A northward shift of range margins in British Odonata. Glob. Change Biol., 11, 502-506. [JoC]
Hickling, R., D.B. Roy, J.K. Hill, R. Fox and C.D. Thomas, 2006: The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Change Biol., 12, 450-455. [JoC]
Hilden, M., H. Lethtonen, I. Barlund, K. Hakala, T. Kaukoranta and S. Tattari, 2005: The practice and process of adaptation in Finnish agriculture. FINADAPT Working Paper 5, Finnish Environment Institute Mimeographs 335, Helsinki, 28 pp. [NPR]
Hill, J.K., C.D. Thomas and D.S. Blakeley, 1999a: Evolution of flight morphology in a butterfly that has recently expanded its geographic range. Oecologia, 121, 165-170. Clean
Hill, J.K., C.D. Thomas and B. Huntley, 1999b: Climate and habitat availability determine 20th-century changes in a butterfly’s range margin. P. Roy. Soc. Lond. B Bio., 266, 1197. Clean
Hill, J.K., Y.C. Collingham, C.D. Thomas, D.S. Blakeley, R. Fox, D. Moss and B. Huntley, 2001: Impacts of landscape structure on butterfly range expansion. Ecol. Lett., 4, 313. Clean
Hock, R., 2005: Glacier melt: a review on processes and their modelling. Prog. Phys. Geog., 29, 362-391. [MoS]
Hock, R., P. Jansson and L. Braun, 2005: Modelling the response of mountain glacier discharge to climate warming. Global Change and Mountain Regions: An Overview of Current Knowledge, U.M. Huber, H.K.M. Bugmann and M.A. Reasoner, Eds., Advances in Global Change Research Series, Springer Netherlands, Dordrecht, 243-254. [NPR]
Hodgkins, G.A., R.W. Dudley and T.G. Huntington, 2003: Changes in the timing of high river flows in New England over the 20th century. J. Hydrol., 278, 244-252. Clean
Hoegh-Guldberg, O., 1999: Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshwater Res., 50, 839-866. [MoS, ARC]
Holbrook, S.J., R.J. Schmitt and J.S. Stephens, 1997: Changes in an assemblage of temperate reef fishes associated with a climate shift. Ecol. Appl., 7, 1299-1310. Clean
Holgate, S.J. and P.L. Woodworth, 2004: Evidence for enhanced coastal sea-level rise during the 1990s. Geophys. Res. Lett., 31, L07305, doi:10.1029/2004GL019626. [JoC]
Holtmeier, F.K. and G. Broll, 2005: Sensitivity and response of northern hemisphere altitudinal and polar treelines to environmental change at landscape and local scales. Global Ecol. Biogeogr., 14, 395-410. Clean
Hubalek, Z., 2003: Spring migration of birds in relation to North Atlantic Oscillation. Folia Zool., 52, 287-298. Clean
Huggel, C., W. Haeberli, A. Kaab, D. Bieri and S. Richardson, 2004: An assessment procedure for glacial hazards in the Swiss Alps. Can. Geotech. J., 41, 1068-1083. [SRC]
Hughes, C.L., J.K. Hill and C. Dytham, 2003a: Evolutionary trade-offs between reproduction and dispersal in populations at expanding range boundaries. P. Roy. Soc. Lond. B Bio, 270, S147. Clean
Hughes, L. 2000: Biological consequences of global warming: is the signal already apparent? Trends Ecol. Evol., 15, 56-61. [ARC]
Hughes, T.P., A.H. Baird, D.R. Bellwood, M. Card, S.R. Connolly, C. Folke R. Grosberg, O. Hoegh-Guldberg, J.B.C. Jackson, J. Kleypas, J.M. Lough, P. Marshall, M. Nystrom, S.R. Palumbi, J.M. Pandolfi, B. Rosen, B. and J. Roughgarden, 2003b: Climate change, human impacts, and the resilience of coral reefs. Science, 301, 929-933. [JoC, SRC]
Huntington, H. and S. Fox, 2004: The changing Arctic: indigenous perspectives. Arctic Climate Impact Assessment, ACIA, Cambridge University Press, Cambridge, 62-98. [NPR]
Huntington, T.G., 2006: Evidence for intensification of the global water cycle: review and synthesis. J. Hydrol., 319, 83-95. Clean
Huntington, T.G., G.A. Hodgkins and R.W. Dudley, 2003: Historical trends in river ice thickness and coherence in hydroclimatological trends in Maine. Climatic Change, 61, 217-236. [JoC]
Huppop, O. and K. Huppop, 2003: North Atlantic Oscillation and timing of spring migration in birds. P. Roy. Soc. Lond. B Bio., 270, 233-240. Clean
Hussell, D.J.T., 2003: Climate change, spring temperatures and timing of breeding of tree swallows (Tachycineta bicolor) in southern Ontario. Auk, 120, 607-618. Clean
Huynen, M., P. Martens, D. Schram, M. Weijenberg and A.E. Kunst, 2001: The impact of cold spells and heatwaves on mortality rates in the Dutch population. Environ. Health Perspect., 109, 463-470. Clean
Inouye, D.W., B. Barr, K.B. Armitage and B.D. Inouye, 2000: Climate change is affecting altitudinal migrants and hibernating species. P. Natl. Acad. Sci. USA, 97, 1630. [JoC]
Inouye, D.W., M.A. Morales and G.J. Dodge, 2002: Variation in timing and abundance of flowering by Delphinium barbeyi Huth (Ranunculaceae): the roles of snowpack, frost and La Niña, in the context of climate change. Oecologia, 130, 543-550. Clean
IPCC, 2001a: Climate Change 2001: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change, J.J. McCarthy, O.F. Canziani, N.A. Leary, D.J. Dokken and K.S. White, Eds., Cambridge University Press, Cambridge, 1032 pp. [NPR]
IPCC, 2001b: Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change, J.T. Houghton, Y. Ding, D.J. Griggs, M. Noguer, P.J. van der Linden, X. Dai, K. Maskell and C.A. Johnson, Eds., Cambridge University Press, Cambridge, 881 pp. [NPR]
IPCC, 2003: IPCC Workshop Report on the Detection and Attribution of the Effects of Climate Change, C. Rosenzweig and P.G. Neofotis, Eds., NASA/Goddard Institute for Space Studies, New York, 87 pp. [NPR]
IPCC, 2007: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller, Eds., Cambridge University Press, Cambridge, 996 pp. [NPR, 2007]
Jansen, E., J. Overpeck, K.R. Briffa, J.-C. Duplessy, F. Joos, V. Masson-Delmotte, D.O. Olago, B. Otto-Bliesner, W.R. Peltier, S. Rahmstorf, R. Ramesh, D. Raynaud, D.H. Rind, O. Solomina, R. Villalba and D. Zhang, 2007: Paleoclimate. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller, Eds., Cambridge University Press, Cambridge, 433-498. [NPR, PoC, 2007]
Jansson, P., R. Hock and T. Schneider, 2003: The concept of glacier storage: a review. J. Hydrol., 282, 116-129. Clean
Jarvinen, A., 1994: Global warming and egg size of birds. Ecography, 17, 108. Clean
Jarvinen, A., 1996: Correlation between egg size and clutch size in the pied flycatcher Ficedula hypoleuca in cold and warm summers. Ibis, 138, 620-623. Clean
Johns, D.G., M. Edwards and S.D. Batten, 2001: Arctic boreal plankton species in the Northwest Atlantic. Can. J. Fish. Aquat. Sci., 58, 2121-2124. [SRC]
Johns, D.G., M. Edwards, A. Richardson and J.I. Spicer, 2003: Increased blooms of a dinoflagellate in the NW Atlantic. Mar. Ecol.–Prog. Ser., 265, 283-287. [SRC]
Johnson, H., S. Kovats, G. McGregor, J. Stedman, M. Gibbs, H. Walton and L. Cook, 2004: The impact of the 2003 heat wave on mortality and hospital admissions in England. Epidemiology, 15, S126-S126. [ARC]
Jones, G.A. and G.H.R. Henry, 2003: Primary plant succession on recently deglaciated terrain in the Canadian High Arctic. J. Biogeogr., 30, 277-296. [ARC]
Jones, G.V., 2005: Climate change in the western United States grape growing regions. 7th International Symposium on Grapevine Physiology and Biotechnology, 689, 71-80. Clean
Jones, G.V. and R.E. Davis, 2000: Climate influences on grapevine phenology, grape composition and wine production and quality for Bordeaux, France. Am. J. Enol. Viticult., 51, 249-261. Clean
Jones, I., F. Hunter and G. Roberston, 2002: Annual adult survival of Least Auklets (Aves, Alcidae) varies with large-scale climatic conditions of the North Pacific Ocean. Oecologia, 133, 38-44. Clean
Jordan, E., 1991: Die gletscher der bolivianischen Anden: eine photogrammetrisch-kartographische Bestandsaufnahme der Gletscher Boliviens als Grundlage für klimatische Deutungen und Potential für die wirtschaftliche Nutzung (The glaciers of the Bolivian Andes, a photogrammetric-cartographical inventory of the Bolivian glaciers as a basis for climatic interpretation and potential for economic use). Erdwissenschaftliche Forschung 23, Franz Steiner Verlag, Stuttgart, 401 pp. [NPR]
Juanes, F., S. Gephard and K. Beland, 2004: Long-term changes in migration timing of adult Atlantic salmon (Salmo salar) at the southern edge of the species distribution. Can. J. Fish. Aquat. Sci., 61, 2392-2400. Clean
Juen, I., G. Kaser and C. Georges, 2007: Modelling observed and future runoff from a glacierized tropical catchment (Cordillera Blanca, Perú). Global Planet. Change, doi:10.1016/j.gloplacha.2006.11.038. [NPR, SRC, 2007]
Jump, A.S., J.M. Hunt and J. Peñuelas, 2006: Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Change Biol., 12, 2163-2174. [NPR]
Kaab, A., C. Huggel, L. Fischer, S. Guex, F. Paul, I. Roer, N. Salzmann, S. Schlaefli, K. Schmutz, D. Schneider, T. Strozzi and Y. Weidmann, 2005: Remote sensing of glacier- and permafrost-related hazards in high mountains: an overview. Nat. Hazard. Earth Sys., 5, 527-554. Clean
Kalnay, E. and M. Cai, 2003: Impact of urbanization and land-use change on climate. Nature, 423, 528-531. [JoC]
Kanuscak, P., M. Hromada, P. Tryjanowski and T. Sparks, 2004: Does climate at different scales influence the phenology and phenotype of the river warbler Locustella fluviatilis? Oecologia, 141, 158-163. [SRC]
Karban, R. and S.Y. Strauss, 2004: Physiological tolerance, climate change and a northward range shift in the spittlebug, Philaenus spumarius. Ecol. Entomol., 29, 251-254. [NPR]
Karl, T.H. and R.W. Knight, 1997: The 1995 Chicago heat wave: how likely is a recurrence? B. Am. Meteorol. Soc., 78, 1107-1119. [JoC]
Karl, T.R. and K.E. Trenberth, 2003: Modern global climate change. Science, 302, 1719-1723. [PoC, JoC]
Karoly, D.J. and Q. Wu, 2005: Detection of regional surface temperature trends. J. Climate, 18, 4337-4343. [JoC, SRC]
Karoly, D.J. and P.A. Stott, 2006: Anthropogenic warming of central England temperature. Atmos. Sci. Lett., 7, 81-85. [SRC]
Karst-Riddoch, T.L., M.F.J. Pisaric and J.P. Smol, 2005: Diatom responses to 20th century climate-related environmental changes in high-elevation mountain lakes of the northern Canadian Cordillera. J. Paleolimnol., 33, 265-282. Clean
Kaser, G. and H. Osmaston, 2002. Tropical Glaciers. Cambridge University Press, Cambridge, 227 pp. [NPR, SRC]
Kearney, M., A. Rogers, J. Townshend, E. Rizzo, D. Stutzer, J. Stevenson and K. Sundborg, 2002: Landsat imagery shows decline of coastal marshes in Chesapeake and Delaware Bays. EOS Transactions, 83, 173, doi:10.1029/2002EO000112. Clean
Keeling, C.D., J.F.S. Chin and T.P. Whorf, 1996: Increased activity of northern vegetation inferred from atmospheric CO2 measurements. Nature, 382, 146-149. [JoC]
Keller, F. and C. Korner, 2003: The role of photoperiodism in alpine plant development. Arct. Antarct. Alp. Res., 35, 361-368. [ARC]
Kennish, M.J., 2001: Coastal salt marsh systems in the US: a review of anthropogenic impacts. J. Coastal Res., 17, 731-748. Clean
Klanderud, K. and H.J.B. Birks, 2003: Recent increases in species richness and shifts in altitudinal distributions of Norwegian mountain plants. Holocene, 13, 1-6. Clean
Klasner, F.L. and D.B. Fagre, 2002: A half century of change in alpine treeline patterns at Glacier National Park, Montana, USA. Arct. Antarct. Alp. Res., 34, 49-56. Clean
Kleypas, J.A., R.W. Buddemeier, D. Archer, J.P. Gattuso, C. Langdon and B.N. Opdyke, 1999: Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science, 284, 118-120. [JoC]
Knutson, T.R., T.L. Delworth, K.W. Dixon, I.M. Held, J. Lu, V. Ramaswamy, M.D. Schwarzkopf, G. Stenchikov and R.J. Stouffer, 2006: Assessment of twentieth century regional surface air temperature trends using the GFDL CM2 coupled models. J. Climate, 19, 1624-1651. [JoC, MoS]
Konvicka, M., M. Maradova, J. Benes, Z. Fric and P. Kepka, 2003: Uphill shifts in distribution of butterflies in the Czech Republic: effects of changing climate detected on a regional scale. Global Ecol. Biogeogr., 12, 403-410. Clean
Korhola, A., S. Sorvari, M. Rautio, P.G. Appleby, J.A. Dearing, Y. Hu, N. Rose, A. Lami and N.G. Cameron, 2002: A multi-proxy analysis of climate impacts on the recent development of subarctic Lake Saanajarvi in Finnish Lapland. J. Paleolimnol., 28, 59-77. Clean
Kosatsky, T., 2005: The 2003 European heat waves. Euro Surveillance, 10, 148-149. Clean
Kovats, R.S., D.H. Campbell-Lendrum, A.J. McMichael, A. Woodward and J.S. Cox, 2001: Early effects of climate change: do they include changes in vector-borne disease? Philos. T. Roy. Soc. B, 356, 1057-1068. [JoC, ARC]
Kovats, R.S., M.J. Bouma, S. Hajat, E. Worrall and A. Haines, 2003: El Niño and health. Lancet, 362, 1481-1489. Clean
Kozlov, M.V. and N.G. Berlina, 2002: Decline in length of the summer season on the Kola Peninsula, Russia. Climatic Change, 54, 387-398. [JoC]
Krupnik, I. and D. Jolly, Eds., 2002: The Earth is Faster Now: Indigenous Observations of Arctic Environmental Change. Arctic Research Consortium of the United States, Fairbanks, 356 pp. [NPR]
Kullman, L., 2002: Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. J. Ecol., 90, 68-77. Clean
Kundzewicz, Z., 2004: Detection of change in world-wide hydrological time series of maximum annual flow. GRDC Report 32, 36 pp. [NPR, ARC]
Kundzewicz, Z.W., U. Ulbrich, T. Brucher, D. Graczyk, A. Kruger, G.C. Leckebusch, L. Menzel, I. Pinskwar, M. Radziejewski and M. Szwed, 2005: Summer floods in central Europe: climate change track? Nat. Hazards, 36, 165-189. [ARC]
Kunst, A.E., C.W.N. Looman and J.P. Mackenbach, 1991: The decline in winter excess mortality in the Netherlands. Int. J. Epidemiol., 20, 971-977. Clean
Kysely, J., 2004: Mortality and displaced mortality during heat waves in the Czech Republic. Int. J. Biometeorol., 49, 91-97. Clean
Labat, D., Y. Godderis, J.L. Probst and J.L. Guyot, 2004: Evidence for global runoff increases related to climate warming. Adv. Water Resour., 27, 631-642. Clean
Ladenburger, C.G., A.L. Hild, D.J. Kazmer and L.C. Munn, 2006: Soil salinity patterns in Tamarix invasions in the Bighorn Basin, Wyoming, USA. J. Arid Environ., 65, 111-128. Clean
Laing, T.E. and J.P. Smol, 2003: Late Holocene environmental changes inferred from diatoms in a lake on the western Taimyr Peninsula, northern Russia. J. Paleolimnol., 30, 231-247. Clean
Lam, J.C., C.L. Tsang and D.H.W. Li, 2004: Long term ambient temperature analysis and energy use implications in Hong Kong. Energ. Convers. Manage., 45, 315-327. [SRC]
Lammers, R.B., A.I. Shiklomanov, C.J. Vorosmarty, B.M. Fekete and B.J. Peterson, 2001: Assessment of contemporary Arctic river runoff based on observational discharge records. J. Geophys. Res.–Atmos., 106(D4), 3321-3334. Clean
Landsea, C.W., 2005: Meteorology: hurricanes and global warming. Nature, 438, E11-E13. [JoC]
Landsea, C.W., R.A. Pielke Jr., A.M. Mestas-Nuñez and J.A. Knaff, 1999: Atlantic basin hurricanes: indices of climatic change. Climatic Change, 42, 89-129. [JoC]
Langdon, C., T. Takahashi, C. Sweeney, D. Chipman, J. Goddard, F. Marubini, H. Aceves, H. Barnett and M.J. Atkinson, 2000: Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Global Biogeochem. Cy., 14, 639-654. Clean
Lantuit, H. and W.H. Pollard, 2003: Remotely sensed evidence of enhanced erosion during the 20th century on Herschel Island, Yukon Territory. Ber. Polar. Meeresforsch., 443, 54-59. [JoC]
Larsen, C.F., R.J. Motyka, J.T. Freymueller, K.A. Echelmeyer and E.R. Ivins, 2005: Rapid uplift of southern Alaska caused by recent ice loss. Geophys. J. Int., 158, 1118-1133. Clean
Larsson, N., 2003: Adapting to climate change in Canada. Build. Res. Inf., 31, 231-239. Clean
Laternser, M. and M. Schneebeli, 2003: Long-term snow climate trends of the Swiss Alps (1931-99). Int. J. Climatol., 23, 733-750. [JoC]
Lavaniegos, B.E. and M.D. Ohman, 2003: Long-term changes in pelagic tunicates of the California Current. Deep-Sea Res. Pt. II, 50, 2473-2498. Clean
Lawrence, D.M. and A.G. Slater, 2005: A projection of severe near-surface permafrost degradation during the 21st century. Geophys. Res. Lett., 32, L24401, doi:10.1029/2005GL025080. [JoC, MoS]
Lawson, P.W., E.A. Logerwell, N.J. Mantua, R.C. Francis and V.N. Agostini, 2004: Environmental factors influencing freshwater survival and smolt production in Pacific Northwest coho salmon (Oncorhynchus kisutch). Can. J. Fish. Aquat. Sci., 61, 360-373. Clean
Leatherman, S.P., K. Zhang and B.C. Douglas, 2000: Sea-level rise shown to drive coastal erosion. EOS Transactions, 81, 55-57. Clean
Le Treut, H., R. Somerville, U. Cubasch, Y. Ding, C. Mauritzen, A. Mokssit, T. Peterson and M. Prather, 2007: Historical overview of climate change science. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller, Eds., Cambridge University Press, Cambridge, 93-128. [NPR, 2007]
Ledneva, A., A.J. Miller-Rushing, R.B. Primack and C. Imbres, 2004: Climate change as reflected in a naturalist’s diary, Middleborough, Massachusetts. Wilson Bull., 116, 224-231. Clean
Lee, C.S., X.D. Li, W.Z. Shi, S.C. Cheung and I. Thornton, 2006: Metal contamination in urban, suburban and country park soils of Hong Kong: a study based on GIS and multivariate statistics. Sci. Total Environ., 356, 45-61. Clean
Legates, D.R., H.F. Lins and G.J. McCabe, 2005: Comments on “Evidence for global runoff increase related to climate warming” by Labat et al. Adv. Water Resour., 28, 1310-1315. Clean
Legendre, L. and R.B. Rivkin, 2002: Pelagic food webs: responses to environmental processes and effects on the environment. Ecol. Res., 17, 143-149. Clean
Lehikoinen, E., T.H. Sparks and M. Zalakevicius, 2004: Arrival and departure dates. Adv. Ecol. Res., 35, 1-31. [SRC]
Lemke, P., J. Ren, R. Alley, I. Allison, J. Carrasco, G. Flato, Y. Fujii, G. Kaser, P. Mote, R. Thomas and T. Zhang, 2007: Observations: Changes in Snow, Ice and Frozen Ground. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller, Eds., Cambridge University Press, Cambridge, 337-384. [NPR, SRC, 2007]
Lepers, E., E.F. Lambin, A.C. Janetos, R. DeFries, F. Achard, N. Ramankutty and R.J. Scholes, 2004: A synthesis of information on rapid land-cover change for the period 1981-2000. BioScience, 55, 115-124. [ARC]
Lesica, P. and B. McCune, 2004: Decline of arctic-alpine plants at the southern margin of their range following a decade of climatic warming. J. Veg. Sci., 15, 679-690. Clean
Levermore, G.J. and E. Keeble, 1998: Dry-bulb temperature analyses for climate change at three UK sites in relation to the forthcoming CIBSE Guide to Weather and Solar Data. Building Services Engineering Research and Technology, 19, 175-181. Clean
Levetin, E., 2001: Effects of climate change on airborne pollen. J. Allergy Clin. Immun., 107, S172-S172. Clean
Levine, M., D. Ürge-Vorsatz, K. Blok, L. Geng, D. Harvey, S. Lang, G. Levermore, A. Mongameli Mehlwana, S. Mirasgedia, A. Novikovam, J. Rilling and H. Yoshino, 2007: Residential and commercial buildings. Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, B. Metz, O. Davidson, P.Bosch, R. Dave and L. Meyer, Eds., Cambridge University Press, Cambridge, UK. [NPR, 2007]
Linderholm, H.W., 2006: Growing season changes in the last century. Agr. Forest Meteorol., 137, 1-14. Clean
Lindgren, E. and R. Gustafson, 2001: Tick-borne encephalitis in Sweden and climate change. Lancet, 358, 16-18. Clean
Lindgren, E., L. Talleklint and T. Polfeldt, 2000: Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Persp., 108, 119-123. [NPR]
Lionello, P., 2005: Extreme surges in the Gulf of Venice: present and future climate. Flooding and Environmental Challenges for Venice and its Lagoon: State of Knowledge 2003, C. Fletcher and T. Spencer, Eds., Cambridge University Press, Cambridge, 59-70. [NPR]
Lionello, P. and A. Sanna, 2005: Mediterranean wave climate variability and its links with NAO and Indian Monsoon. Clim. Dynam., 25, 611-623. [JoC]
Lise, W. and R.S.J. Tol, 2002: Impact of climate on tourist demand. Climatic Change, 55, 429-449. [JoC, ARC]
Livingstone, D.M. and M.T. Dokulil, 2001: Eighty years of spatially coherent Austrian lake surface temperatures and their relationship to regional air temperature and the North Atlantic Oscillation. Limnol. Oceanogr., 46, 1220-1227. Clean
Lobell, D.B. and G.P. Asner, 2003: Climate and management contributions to recent trends in U.S. agricultural yields. Science, 299, 1032. [JoC]
Lobo, A. and P. Maisongrande, 2006: Stratified analysis of satellite imagery of SW Europe during summer 2003: the differential response of vegetation classes to increased water deficit. Hydrol. Earth Syst. Sc., 10, 151-164. Clean
Lorch, R., 1990: Energy and global responsibility. RIBA J., March, 50-51. Clean
Lorke, A., K. Tietze, M. Halbwachs and A. Wuest, 2004: Response of Lake Kivu stratification to lava inflow and climate warming. Limnol. Oceanogr., 49, 778-783. Clean
Lotsch, A.M., A. Friedl, B.T. Anderson and C.J. Tucker, 2005: Response of terrestrial ecosystems to recent Northern Hemispheric drought. Geophys. Res. Lett., 32, L06705, doi:10.1029/2004GL022043. [JoC]
Lucht, W., I.C. Prentice, R.B. Myneni, S. Sitch, P. Friedlingstein, W. Cramer, P. Bousquet, W. Buermann and B. Smith, 2002: Climatic control of the high-latitude vegetation greening trend and Pinatubo effect. Science, 296, 1687-1689. [JoC, ARC]
Luterbacher, J., D. Dietrich, E. Xoplaki, M. Grosjean and H. Wanner, 2004: European seasonal and annual temperature variability, trends and extremes since 1500. Science, 303, 1499-1503. [JoC]
MacDonald, M., L. Arragutainaq and Z. Novalinga, 1997: Voices from the Bay: Traditional Ecological Knowledge of Inuit and Cree. Canadian Arctic Resource Committee and Environmental Committee of Municipality of Sanikiluaq, Ottawa, 98 pp. [NPR]
Maddison, D., 2001: In search of warmer climates? The impact of climate change on flows of British tourists. Climatic Change, 49, 193-208. [JoC]
Mark, B.G., J.M. McKenzie and J. Gómez, 2005: Hydrochemical evaluation of changing glacier meltwater contribution to stream discharge: Callejon de Huaylas, Peru. Hydrolog. Sci. J, 50, 975-987. Clean
Masek, J.G., 2001: Stability of boreal forest stands during recent climate change: evidence from Landsat satellite imagery. J. Biogeogr., 28, 967-976. Clean
Maselli, F., 2004: Monitoring forest conditions in a protected Mediterranean coastal area by the analysis of multi-year NDVI data. Remote Sens. Environ., 89, 423-433. Clean
Matsumoto, K., T. Ohta, M. Irasawa and T. Nakamura, 2003: Climate change and extension of the Ginkgo biloba L. growing season in Japan. Glob. Change Biol., 9, 1634-1642. [JoC]
McCabe, G. and M.P. Clark, 2005: Trends and variability in snowmelt runoff in the western United States. J. Hydrometeorol., 6, 476-482. [JoC]
McCallum, H., D. Harvell and A. Dobson, 2003: Rates of spread of marine pathogens. Ecol. Lett., 6, 1062-1067. Clean
McGowan, J.A., D.R. Cayan and L.M. Dorman, 1998: Climate-ocean variability and ecosystem response in the northeast Pacific. Science, 281, 210-217. [JoC, ARC]
McKenzie, D., Z. Gedalof, D.L. Peterson and P. Mote, 2004, Climatic change, wildfire and conservation. Conserv. Biol., 18, 890-902. Clean
McLaughlin, J.F., J.J. Hellmann, C.L. Boggs and P.R. Ehrlich, 2002a: Climate change hastens population extinctions. P. Natl. Acad. Sci. USA, 99, 6070-6074. [JoC]
McLaughlin, J.F., J.J. Hellmann, C.L. Boggs and P.R. Ehrlich, 2002b: The route to extinction: population dynamics of a threatened butterfly. Oecologia, 132, 538-548. Clean
McNamara, J.P., D.L. Kane and L.D. Hinzman, 1999: An analysis of an arctic channel network using a digital elevation model. Geomorphology, 29, 339-353. [MoS]
McWilliams, J.P., I.M. Côté, J.A. Gill, W.J. Sutherland and A.R. Watkinson, 2005: Accelerating impacts of temperature-induced coral bleaching in the Caribbean. Ecology, 86, 2055-2060. [SRC]
Mendes, J.M. and J.D. Woodley, 2002: Effect of the 1995-1996 bleaching event on polyp tissue depth, growth, reproduction and skeletal band formation in Montastraea annularis. Mar. Ecol.–Prog. Ser., 235, 93-102. [NPR]
Menendez, R., A.G. Megias, J.K. Hill, B. Braschler, S.G. Willis, Y. Collingham, R. Fox, D.B. Roy and C.D. Thomas, 2006: Species richness changes lag behind climate change. P. Roy. Soc. Lond. B Bio., 273, 1465-1470. Clean
Menzel, A., 2000: Trends in phenological phases in Europe between 1951 and 1996. Int. J. Biometeorol., 44, 76-81. [SRC]
Menzel, A., 2002: Phenology: its importance to the global change community. Climatic Change, 54, 379-385. [JoC, SRC]
Menzel, A., 2003: Plant phenological anomalies in Germany and their relation to air temperature and NAO. Climatic Change, 57, 243-263. [JoC, SRC]
Menzel, A. and P. Fabian, 1999: Growing season extended in Europe. Nature, 397, 659-659. [JoC, SRC]
Menzel, A. and N. Estrella, 2001: Plant phenological changes. “Fingerprints” of Climate Change: Adapted Behaviour and Shifting Species Ranges, G.-R. Walther, C.A. Burga and P.J. Edwards, Eds., Kluwer Academic/Plenum, New York, 123-137. [NPR, SRC]
Menzel, A. and V. Dose, 2005: Analysis of long-term time series of the beginning of flowering by Bayesian function estimation. Meteorol. Z., 14, 429-434. [MoS, SRC]
Menzel, A., N. Estrella and P. Fabian, 2001: Spatial and temporal variability of the phenological seasons in Germany from 1951 to 1996. Glob. Change Biol., 7, 657-666. [JoC, SRC]
Menzel, A., G. Jakobi, R. Ahas, H. Scheifinger and N. Estrella, 2003: Variations of the climatological growing season (1951-2000) in Germany compared with other countries. Int. J. Climatol., 23, 793-812. [JoC, SRC]
Menzel, A., A. Estrella and A. Testka, 2005a: temperature response rates from long-term phenological records. Climate Res., 30, 21-28. [SRC]
Menzel, A., T.H. Sparks, N. Estrella and S. Eckhardt, 2005b: ‘SSW to NNE’: North Atlantic Oscillation affects the progress of seasons across Europe. Glob. Change Biol., 11, 909-918. [JoC, SRC]
Menzel, A., T. Sparks, N. Estrella and D.B. Roy, 2006a: Geographic and temporal variability in phenology. Global Ecol. Biogeogr., 15, 498-504. [SRC]
Menzel, A., T.H. Sparks, N. Estrella, E. Koch, A. Aasa, R. Ahas, K. Alm-Kübler, P. Bissolli, O. Braslavská, A. Briede, F.M. Chmielewski, Z. Crepinsek, Y. Curnel, Å. Dahl, C. Defila, A. Donnelly, Y. Filella, K. Jatczak, F. Måge, A. Mestre, Ø. Nordli, J. Peñuelas, P. Pirinen, V. Remi?ová, H. Scheifinger, M. Striz, A. Susnik, A.J.H. van Vliet, F.-E. Wielgolaski, S. Zach and A. Zust, 2006b: European phenological response to climate change matches the warming pattern. Glob. Change Biol., 12, 1969-1976. [JoC, SRC]
Menzel, A., J. von Vopelius, N. Estrella, C. Schleip and V. Dose, 2006c: Farmers’ annual activities are not tracking speed of climate change. Climate Res., 32, 201-207. [SRC]
Meshinev, T., I. Apostolova and E. Koleva, 2000: Influence of warming on timberline rising: a case study on Pinus peuce Griseb. in Bulgaria. Phytocoenologia, 30, 431-438. Clean
Mestas-Nunez, A.M. and A.J. Miller, 2006: Interdecadal variability and climate change in the eastern tropical Pacific: a review. Prog. Oceanogr., 69, 267-284. Clean
Michelutti, N., M.S.V. Douglas and J.P. Smol, 2003: Diatom response to recent climatic change in a high arctic lake (Char Lake, Cornwallis Island, Nunavut). Global Planet. Change, 38, 257-271. Clean
Middleton, E.M., J.R. Herman, E.A. Celarier, J.W. Wilkinson, C. Carey and R.J. Rusin, 2001: Evaluating ultraviolet radiation exposure with satellite data at sites of amphibian declines in Central and South America. Conserv. Biol., 15, 914-929. Clean
Millar, J.S. and E.J. Herdman, 2004: Climate change and the initiation of spring breeding by deer mice in the Kananaskis Valley, 1985-2003. Can. J. Zool., 82, 1444-1450. Clean
Milly, P.C.D., R.T. Wetherald, K.A. Dunne and T.L. Delworth, 2002: Increasing risk of great floods in a changing climate. Nature, 415, 514-517. [JoC]
Milly, P.C.D., K.A. Dunne and A.V. Vecchia, 2005: Global pattern of trends in streamflow and water availability in a changing climate. Nature, 438, 347-350. [JoC]
Mimura, N. and P.D. Nunn, 1998: Trends of beach erosion and shoreline protection in rural Fiji. J. Coastal Res., 14, 37-46. [ARC]
Mitchell, J.F.B., D.J. Karoly, M.R. Allen, G. Hegerl, F. Zwiers and J. Marengo, 2001: Detection of climate change and attribution of causes. Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change, J.T. Houghton, Y. Ding, D.J. Griggs, M. Noguer, P.J. van der Linden, X. Dai, K. Maskell and C.A. Johnson, Eds., Cambridge University Press, Cambridge, 695-738. [NPR, SRC]
Mohr, J.J. and R. Forsberg, 2002: Remote sensing: searching for new islands in sea ice: coastlines concealed in polar seas are now more accessible to cartography. Nature, 416, 35-35. [JoC]
Moiseev, P.A. and S.G. Shiyatov, Eds., 2003: Vegetation dynamics at the treeline ecotone in the Ural highlands, Russia. Alpine Biodiversity in Europe: A Europe-wide Assessment of Biological Richness and Change, L. Nagy, G. Grabherr, C. Körner and D.B.A. Thompson, Eds., Ecological Studies 167, Springer, Berlin, 423-435. [NPR]
Mölg, T., D.R. Hardy, N. Cullen and G. Kaser, 2005: Tropical glaciers in the context of climate change and society: focus on Kilimanjaro (East Africa). Contribution to Mountain Glaciers and Society Workshop. California University Press, Wengen, 28 pp. [NPR, SRC]
Molyneux, D., 2003: Climate change and tropical disease: common themes in changing vector-borne disease scenarios. T. Roy. Soc. Trop Med. H., 97, 129-32. Clean
Mool, P.K., D. Wangda and S.R. Bajracharya, 2001: Inventory of Glaciers, Glacial Lakes and Glacial Lake Outburst Floods: Monitoring and Early Warning Systems in the Hindu Kush-Himalayan Region. ICIMOD, Bhutan, Kathmandu, 227 pp. [NPR]
Moonen, A.C., L. Ercoli, M. Mariotti and A. Masoni, 2002: Climate change in Italy indicated by agrometeorological indices over 122 years. Agr. Forest Meteorol., 111, 13-27. Clean
Mueller, D.R., W.F. Vincent and M.O. Jeffries, 2003: Break-up of the largest Arctic ice shelf and associated loss of an epishelf lake. Geophys. Res. Lett., 30, 2031, doi:10.1029/2003GL017931. [JoC]
Muir Wood, R., S. Miller and A. Boissonnade, 2006: The search for trends in a global catalogue of normalized weather-related catastrophe losses. Workshop on Climate Change and Disaster Losses: Understanding and Attributing Trends and Projections. Hohenkammer, Munich, 188-194. [NPR, MoS]
Munich Re Group, 2005: Annual Review: Natural Catastrophes 2004. WKD Offsetdruck GmbH, Munich, 60 pp. [NPR]
Myneni, R.B., C.D. Keeling, C.J. Tucker, G. Asrar and R.R. Nemani, 1997: Increased plant growth in the northern high latitudes from 1981 to 1991. Nature, 386, 698-702. [JoC]
Nabuurs, G.J., A. Pussinen, T. Karjalainen, M. Erhard and K. Kramer, 2002: Stemwood volume increment changes in European forests due to climate change: a simulation study with the EFISCEN model. Glob. Change Biol., 8, 304-316. [JoC, MoS]
Nelson, F.E., 2003: (Un)frozen in time. Science, 299, 1673-1675. [JoC, ARC]
Nemani, R.R., M.A. White, D.R. Cayan, G.V. Jones, S.W. Running, J.C. Coughlan and D.L. Peterson, 2001: Asymmetric warming over coastal California and its impact on the premium wine industry. Climate Res., 19, 25-34. [ARC]
Nemani, R.R., C.D. Keeling, H. Hashimoto, W.M. Jolly, S.C. Piper, C.J. Tucker, R.B. Myneni and S.W. Running, 2003: Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science, 300, 1560-1563. [JoC, ARC]
Newman, J.A., 2005: Climate change and the fate of cereal aphids in Southern Britain. Glob. Change Biol., 11, 940-944. [JoC]
Nicol, F., 2004: Adaptive thermal comfort standards in the hot-humid tropics. Energ. Buildings, 36, 628-637. Clean
NOAA, 2005: Preliminary Report: Hurricane Katrina storm tide summary. Center for Operational Oceanographic Products and Services, U.S. Department of Commerce National Ocean Service, Silver Spring, Maryland, 20 pp. [NPR]
Noetzli, J., M. Hoelzle and W. Haeberli, 2003: Mountain permafrost and recent Alpine rock-fall events: a GIS-based approach to determine critical factors. 8th International Conference on Permafrost, Zürich, Proceedings Vol. 2, M. Phillips, S. Springman and L. Arenson, Eds., Swets and Zeitlinger, Lisse, 827-832. [NPR, SRC]
Nowakowski, J.J., 2002: Variation of morphometric parameters within the Savi’s warbler (Locustella luscinioides) population in eastern Poland. Ring, 24, 49. Clean
Nyberg, P., E. Bergstand, E. Degerman and O. Enderlein, 2001: Recruitment of pelagic fish in an unstable climate: studies in Sweden’s four largest lakes. Ambio, 30, 559-564. Clean
Nyssen, J., J. Poesen, J. Moeyersons, J. Deckers, M. Haile and A. Lang, 2004: Human impact on the environment in the Ethiopian and Eritrean highlands: a state of the art. Earth-Sci. Rev., 64, 273-320. Clean
O’Reilly, C.M., 2007: The impact of recent global climate change on the East Africa Rift Valley lakes. Earth-Sci. Rev., submitted. [NPR, SRC, 2007]
O’Reilly, C.M., S.R. Alin, P.D. Plisnier, A.S. Cohen and B.A. McKee, 2003: Climate change decreases aquatic ecosystem productivity of Lake Tanganyika, Africa. Nature, 424, 766-768. [JoC, SRC]
OECD, 2007: Climate change in the European Alps. Adapting Winter Tourism and Natural Hazards Management, Shardul Agrawala Ed., OECD Publishing, Paris, 127 pp. [NPR, 2007]
Ohde, S. and M.M.M. Hossain, 2004: Effect of CaCO3 (aragonite) saturation state of seawater on calcification of Porites coral. Geochem. J., 38, 613-621. Clean
Opdam, P. and D. Wascher, 2004: Climate change meets habitat fragmentation: linking landscape and biogeographical scale levels in research and conservation. Biol. Conserv., 117, 285-297. Clean
Orviku, K., J. Jaagus, A. Kont, U. Ratas and R. Rivis, 2003: Increasing activity of coastal processes associated with climate change in Estonia. J. Coastal Res., 19, 364-375. Clean
Ozaki, N., T. Fukushima, H. Harasawa, T. Kojiri, K. Kawashima and M. Ono, 2003: Statistical analyses on the effects of air temperature fluctuations on river water qualities. Hydrol. Process., 17, 2837-2853. [ARC]
Pagano, T. and D. Garen, 2005: A recent increase in western US streamflow variability and persistence. J. Hydrometeorol., 6, 173-179. [JoC]
Pagliosa, P.R. and F.A.R. Barbosa, 2006: Assessing the environment–benthic fauna coupling in protected and urban areas of southern Brazil. Biol. Conserv., 129, 408-417. Clean
Parmesan, C., 2006: Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. S., 37, 637-669. Clean
Parmesan, C. and G. Yohe, 2003: A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421, 37-42. [JoC, ARC]
Parmesan, C. and H. Galbraith, 2004: Observed impacts of global climate change in the US. Report prepared for the Pew Center on Global Climate Change, Washington, District of Columbia, 67 pp. [NPR]
Parmesan, C., N. Rhyrholm, C. Stefanescu, J.K. Hill, C.D. Thomas, H. Descimon, B. Huntley, L. Kalia, J. Kullberg, T. Tammaru, W.J. Tennent, J.A. Thomas and M. Warren, 1999: Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature, 399, 579-583. [JoC]
Parmesan, C., T.L. Root and M.R. Willig, 2000: Impacts of extreme weather and climate on terrestrial biota. B. Am. Meteorol. Soc., 81, 443-450. [JoC, SRC]
Pascual, M., A. Ahumada, L.F. Chaves, X. Rodo and M.J. Bouma, 2006: Malaria resurgence in the East African highlands: temperature trends revisited. P. Natl. Acad. Sci. USA, 103, 5829-5834. [JoC, SRC]
Patz, J.A., M. Hulme, C. Rosenzweig, T.D. Mitchell, R.A. Goldberg, A.K. Githeko, S. Lele, A.J. McMichael and D. Le Sueur, 2002: Climate change: regional warming and malaria resurgence. Nature, 420, 627-628. [JoC, SRC]
Patz, J.A., D. Campbell-Lendrum, T. Holloway and J.A.N. Foley, 2005: Impact of regional climate change on human health. Nature, 438, 310-317. [JoC, ARC]
Pauli, H., M. Gottfried and G. Grabherr, 2001. High summits of the Alps in a changing climate. “Fingerprints” of Climate Change: Adapted Behaviour and Shifting Species Ranges, G.-R. Walther, C.A. Burga and P.J. Edwards, Eds., Kluwer Academic/Plenum, New York, 139-149. [NPR]
Pauli, H., M. Gottfried, T. Dirnbock, S. Dullinger and G. Grabherr, 2003: Assessing the long-term dynamics of endemic plants at summit habitats. Alpine Biodiversity in Europe: A Europe-wide Assessment of Biological Richness and Change, L. Nagy, G. Grabherr, C. Körner and D.B.A. Thompson, Eds., Ecological Studies 167, Springer, Heidelberg, 195-207. [NPR]
Pelejero, C., E. Calvo, M.T. McCulloch, J.F. Marshall, M.K. Gagan, J.M. Lough and B.N. Opdyke, 2005: Preindustrial to modern interdecadal variability in coral reef pH. Science, 309, 2204-2207. [JoC]
Peña, H. and F. Escobar, 1985: Análisis de las crecidas del río Paine, XII Región. Publicación Interna E.H. N. 83/7, 78 pp. [NPR]
Peng, S.B., J.L. Huang, J.E. Sheehy, R.C. Laza, R.M. Visperas, X.H. Zhong, G.S. Centeno, G.S. Khush and K.G. Cassman, 2004: Rice yields decline with higher night temperature from global warming. P. Natl. Acad. Sci. USA, 101, 9971-9975. [JoC]
Penland, S., P.F. Connor, A. Beall, S. Fearnley and S.J. Williams, 2005: Changes in Louisiana’s shoreline: 1855-2002. J. Coastal Res., 44, S7-S39. Clean
Peñuelas, J. and M. Boada, 2003: A global change-induced biome shift in the Montseny mountains (NE Spain). Glob. Change Biol., 9, 131-140. [JoC]
Peñuelas, J., I. Filella and P. Comas, 2002: Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean region. Glob. Change Biol., 8, 531-544. [JoC]
Perren, B.B., R.S. Bradley and P. Francus, 2003: Rapid lacustrine response to recent High Arctic warming: a diatom record from Sawtooth Lake, Ellesmere Island, Nunavut. Arct. Antarct. Alp. Res., 35, 271-278. Clean
Perry, A.L., P.J. Low, J.R. Ellis and J.D. Reynolds, 2005: Climate change and distribution shifts in marine fishes. Science, 308, 1912-1915. [JoC]
Petheram, C., G. Walker, R. Grayson, T. Thierfelder and L. Zhang, 2001: Towards a framework for predicting impacts of land-use on recharge. Aust. J. Soil Res., 40, 397-417. [MoS]
Phillips, O.L., T.R. Baker, L. Arroyo, N. Higuchi, T.J. Killeen, W.F. Laurance, S.L. Lewis, J. Lloyd, Y. Malhi, A. Monteagudo, D.A. Neill, P.N. Vargas, J.N.M. Silva, J. Terborgh, R.V. Martinez, M. Alexiades, S. Almeida, S. Brown, J. Chave, J.A. Comiskey, C.I. Czimczik, A. Di Fiore, T. Erwin, C. Kuebler, S.G. Laurance, H.E.M. Nascimento, J. Olivier, W. Palacios, S. Patino, N.C.A. Pitman, C.A. Quesada, M. Salidas, A.T. Lezama and B. Vinceti, 2004: Pattern and process in Amazon tree turnover, 1976-2001. Philos. T. Roy. Soc. B, 359, 381-407. [JoC]
Pielke, R.A. and C.W. Landsea, 1998: Normalized hurricane damages in the United States: 1925-95. Weather Forecast., 13, 621-631. Clean
Pielke, R.A. and P. Hoppe, 2006: Workshop summary report. Workshop on Climate Change and Disaster Losses: Understanding and Attributing Trends and Projections. Hohenkammer, Munich, 4-12. [NPR, MoS]
Pielke, R.A., Jr., M.W. Downton and J.Z. Barnard Miller, 2002: Flood Damage in the United States, 1926-2000: A Reanalysis of National Weather Service Estimates. National Center for Atmospheric Research (UCAR), Boulder, Colorado, 96 pp. [NPR, MoS]
Pielke, R.A., J. Rubiera, C. Landsea, M.L. Fernandez and R. Klein, 2003: Hurricane vulnerability in Latin America and the Caribbean: normalized damage and loss potentials. Natural Hazards Review, 4, 101-114. [ARC]
Pielke, R.A., C. Landsea, K. Emanuel, M. Mayfield, J. Laver and R. Pasch, 2005: Hurricanes and global warming. B. Am. Meteorol. Soc., 86, 1571. [JoC]
Pilcher, J. and V. Hall, 2001. Flora Hibernica: The Wild Flowers, Plants and Trees of Ireland. Collins Press, Cork, 203 pp. [NPR]
Pirazzoli, P.A., H. Regnauld and L. Lemasson, 2004: Changes in storminess and surges in western France during the last century. Mar. Geol., 210, S307-S323. Clean
Post, E. and M. Forchhammer, 2002: Synchronization of animal population dynamics by large-scale climate. Nature, 420, 168-171. [JoC]
Pounds, J.A., M.R. Bustamante, L.A. Coloma, J.A. Consuegra, M.P.L. Fogden, P.N. Foster, E. La Marca, K.L. Masters, A. Merino-Viteri, R. Puschendorf, S.R. Ron, G.A. Sanchez-Azofeifa, C.J. Still and B.E. Young, 2006: Widespread amphibian extinctions from epidemic disease driven by global warming. Nature, 439, 161-167. [JoC]
Pouyaud, B., M. Zapata, J. Yerren, J. Gomez, G. Rosas, W. Suarez and P. Ribstein, 2005: Avenir des ressources en eau glaciaire de la Cordillère Blanche. Hydrolog. Sci. J., 50, 999-1022. Clean
Power, S., B. Sadler and N. Nicholls, 2005: The influence of climate science on water management in Western Australia: lessons for climate scientists. B. Am. Meteorol. Soc., 86, 839-844. [JoC]
Pretlove, S.E.C. and T. Oreszczyn, 1998: Climate change: impact on the environmental design of buildings. Building Services Engineering Research and Technology, 19, 55-58. Clean
Prospero, J.M. and P.J. Lamb, 2003: African droughts and dust transport to the Caribbean, climate change implications. Science, 302, 1024-27. [JoC]
Prowse, T.D. and S. Beltaos, 2002: Climatic control of river-ice hydrology: a review. Hydrol. Process., 16, 805-822. [ARC]
Psenner, R. and R. Schmidt, 1992: Climate-driven pH control of remote Alpine lakes and effects of acid deposition. Nature, 356, 781-783. [JoC]
Purse, B.V., P.S. Mellor, D.J. Rogers, A.R. Samuel, P.P. Mertens and M. Baylis, 2006: Climate change and the recent emergence of bluetongue in Europe. Nat. Rev. Microbiol., 4, 160. Clean
Quinn, T.P. and D.J. Adams, 1996: Environmental changes affecting the migratory timing of American shad and sockeye salmon. Ecology, 77, 1151-1162. Clean
Raitsos, D.E., P.C. Reid, S.J. Lavender, M. Edwards and A.J. Richardson, 2005: Extending the SeaWiFS chlorophyll data set back 50 years in the northeast Atlantic. Geophys. Res. Lett., 32, L06603. [JoC, SRC]
Ramirez, E., B. Francou, P. Ribstein, M. Descloitres, R. Guerin, J. Mendoza, R. Gallaire, B. Pouyaud and E. Jordan, 2001: Small glaciers disappearing in the tropical Andes: a case-study in Bolivia – Glaciar Chacaltaya (16°S). J. Glaciol., 47, 187-194. [SRC]
Randolph, S.E., 2001: The shifting landscape of tick-borne zoonoses: tick-borne encephalitis and Lyme borreliosis in Europe. Philos. T. Roy. Soc. B, 356, 1045-1056. [JoC]
Rasmussen, A., 2002: The effects of climate change on the birch pollen season in Denmark. Aerobiologia, 18, 253-265. Clean
Reading, C.J., 2003: The effects of variation in climatic temperature (1980-2001) on breeding activity and tadpole stage duration in the common toad, Bufo bufo. Sci. Total Environ., 310, 231-236. [NPR]
Regehr, E.V., S.C. Amstrup and I. Stirling, 2006: Polar bear population status in the southern Beaufort Sea. U.S. Geological Survey Open-File Report 2000-1337, 20 pp. [NPR]
Regonda, S.K., B. Rajagopalan, M. Clark and J. Pitlick, 2005: Seasonal cycle shifts in hydroclimatology over the western United States. J. Climate, 18, 372-384. [JoC]
Reichert, B.K., L. Bengtsson and J. Oerlemans, 2002: Recent glacier retreat exceeds internal variability. J. Climate, 15, 3069-3081. [JoC]
Reid, P.C. and M. Edwards, 2001: Long-term changes in the pelagos, benthos and fisheries of the North Sea. Burning Issues of North Sea Ecology, I. Kröncke, M. Türkay and J. Sündermann, Eds., Senckenbergiana Maritima, 31, 107-115. [NPR]
Reid, P.C., M. Edwards, H.G. Hunt and A.J. Warner, 1998: Phytoplankton change in the North Atlantic. Nature, 391, 546-546. [JoC, SRC]
Restrepo, J.D., B. Kjerfve, I. Correa and J. Gonzalez, 2002: Morphodynamics of a high discharge tropical delta, San Juan River, Pacific coast of Colombia. Mar. Geol., 192, 355-381. Clean
Richardson, S.D. and J.M. Reynolds, 2000. An overview of glacial hazards in the Himalayas. Quatern. Int., 65/66, 31-47. Clean
Richardson, A.J. and D.S. Schoeman, 2004: Climate impact on plankton ecosystems in the Northeast Atlantic. Science, 305, 1609-1612. [JoC]
Rindfuss, R.R., S.J. Walsh, B.L. Turner, J. Fox and V. Mishra, 2004: Developing a science of land change: challenges and methodological issues. P. Natl. Acad. Sci. USA, 101, 13976-13981. [JoC]
Rivera, A., G. Casassa, R. Thomas, E. Rignot, R. Zamora, D. Antúnez, C. Acuña and F. Ordenes, 2005: Glacier wastage on Southern Adelaide Island and its impact on snow runway operations. Ann. Glaciol., 41, 57-62. [SRC]
Robeson, S.M., 2002: Increasing growing-season length in Illinois during the 20th century. Climatic Change, 52, 219-238. [JoC]
Rodo, X., M. Pascual, G. Fuchs and A.S.G. Faruque, 2002: ENSO and cholera: a nonstationary link related to climate change? P. Natl. Acad. Sci. USA, 99, 12901-12906. [JoC, SRC]
Roemmich, D. and J. McGowan, 1995: Climatic warming and the decline of zooplankton in the California current. Science, 267, 1324-1326. [JoC]
Rogers, K., K.M. Wilton and N. Saintilan, 2006: Vegetation change and surface elevation dynamics in estuarine wetlands of southeast Australia. Estuar. Coast. Shelf S., 66, 559-569. Clean
Rogora, M., R. Mosello and S. Arisci, 2003: The effect of climate warming on the hydrochemistry of Alpine lakes. Water Air Soil Pollut., 148, 347-361. Clean
Ron, S.R., W.E. Duellman, L.A. Coloma and M.R. Bustamante, 2003: Population decline of the Jambato toad Atelopus ignescens (Anura: Bufonidae) in the Andes of Ecuador. J. Herpetol., 37, 116-126. Clean
Root, T.L. and S.H. Schneider, 2002. Climate change: overview and implications for wildlife. Wildlife Responses to Climate Change: North American Case Studies, T.L. Root and S.H. Schneider, Eds., Island Press, Washington, District of Columbia, 1-56. [NPR, PoC, SRC]
Root, T.L., J.T. Price, K.R. Hall, S.H. Schneider, C. Rosenzweig and J.A. Pounds, 2003: Fingerprints of global warming on wild animals and plants. Nature, 421, 57-60. [PoC, JoC, SRC]
Root, T.L., D.P. MacMynowski, M.D. Mastrandrea and S.H. Schneider, 2005: Human-modified temperatures induce species changes: Joint attribution. P. Natl. Acad. Sci. USA, 102, 7465-7469. [PoC, JoC, SRC]
Rose, G.A. and R.L. O’Driscoll, 2002: Capelin are good for cod: can the northern stock rebuild without them? ICES J. Mar. Sci., 59, 1018-1026. [ARC]
Rose, J.B., D.E. Huffman and A. Gennaccaro, 2002: Risk and control of waterborne cryptosporidiosis. FEMS Microbiol. Rev., 26, 113-123. Clean
Ross, M.S., J.F. Meeder, J.P. Sah, P.L. Ruiz and G.J. Telesnicki, 2000: The southeast saline everglades revisited: 50 years of coastal vegetation change. J. Veg. Sci., 11, 101-112. Clean
Roy, D.B. and T.H. Sparks, 2000: Phenology of British butterflies and climate change. Glob. Change Biol., 6, 407. [JoC, SRC]
Royal Society, 2005: Ocean Acidification due to Increasing Atmospheric Carbon Dioxide. Royal Society, London, 68 pp. [NPR]
Rudel, T.K., O.T. Coomes, E. Moran, F. Achard, A. Angelsen, J. Xu and E.F. Lambin, 2005: Forest transitions: towards a global understanding of land use change. Global Environ. Chang., 15, 23-31. [JoC]
Ruhland, K., A. Presnitz and J.P. Smol, 2003: Paleolimnological evidence from diatoms for recent environmental changes in 50 lakes across Canadian arctic treeline. Arct. Antarct. Alp. Res., 35, 110-123. Clean
Sagarin, R.D., J.P. Barry, S.E. Gilman and C.H. Baxter, 1999: Climate-related change in an intertidal community over short and long time scales. Ecol. Monogr., 69, 465-490. Clean
Saino, N., M. Romano, R. Ambrosini, R.P. Ferrari and A.P. Moller, 2004: Timing of reproduction and egg quality covary with temperature in the insectivorous barn swallow, Hirundo rustica. Funct. Ecol., 18, 50-57. [NPR]
Saintilan, N. and R.J. Williams, 1999: Mangrove transgression into saltmarsh environments in south-east Australia. Global Ecol. Biogeogr., 8, 117-124. Clean
Sanders, C.H. and M.C. Phillipson, 2003: UK adaptation strategy and technical measures: the impacts of climate change on buildings. Build. Res. Inf., 31, 210-221. Clean
Sanz, J., 2003: Large-scale effects of climate change on breeding parameters of pied flycatchers in Western Europe. Ecography, 26, 45-50. Clean
Sauphanor, B. and T. Boivin, 2004: Changement climatique et résistance du carpocapse aux insecticides. Journées MICCES 2004, 2 pp. [NPR]
Schaber, J., 2002: Phenology in Germany in the 20th century: methods, analyses and models, Dissertation, Department of Geoecology, University of Potsdam, 164 pp. [NPR, MoS]
Schär, C., P.L. Vidale, D. Luthi, C. Frei, C. Haberli, M.A. Liniger and C. Appenzeller, 2004: The role of increasing temperature variability in European summer heatwaves. Nature, 427, 332-336. [JoC]
Scheifinger, H., A. Menzel, E. Koch, C. Peter and R. Ahas, 2002: Atmospheric mechanisms governing the spatial and temporal variability of phenological phases in central Europe. Int. J. Climatol., 22, 1739-1755. [JoC, SRC]
Schindler, D.E., D.E. Rogers, M.D. Scheuerell and C.A. Abrey, 2005: Effects of changing climate on zooplankton and juvenile sockeye salmon growth in southwestern Alaska. Ecology, 86, 198-209. Clean
Schindler, D.E., C. Baldwin, J. Carter, J. Fox, T.B. Francis, S.E. Hampton, G. Holtgrieve, S.P. Johnson, J.W. Moore, W.J. Palen, C. Ruff, J.M. Scheuerell, H. Tallis and M. Winder, 2007: Climate change and responses of freshwater ecosystems. BioScience, submitted. [NPR, 2007]
Schindler, D.W., K.G. Beaty, E.J. Fee, D.R. Cruikshank, E.R. Debruyn, D.L. Findlay, G.A. Linsey, J.A. Shearer, M.P. Stainton and M.A. Turner, 1990: Effects of climatic warming on lakes of the central Boreal Forest. Science, 250, 967-970. [JoC]
Schleip, C., A. Menzel, N. Estrella and V. Dose, 2006: The use of Bayesian analysis to detect recent changes in phenological events throughout the year. Agr. Forest Meteorol., 141, 179-191. [SRC]
Schwartz, M.D. and B.E. Reiter, 2000: Changes in North American spring. Int. J. Climatol., 20, 929-932. [JoC]
Schwartz, M.D. and X. Chen, 2002: Examining the onset of spring in China. Climate Res., 21, 157-164. Clean
Schwörer, D.A., 1997: Bergführer und Klimaänderung: eine Untersuchung im Berninagebiet über mögliche Auswirkungen einer Klimaänderung auf den Bergführerberuf (Mountain guides and climate change: an inquiry into possible effects of climatic change on the mountain guide trade in the Bernina region, Switzerland). Diplomarbeit der philosophisch-naturwissenschaftlichen Fakultät der Universität Bern. [NPR]
Seguin, B., M. Domergue, I.G.D. Cortazar, N. Brisson and D. Ripoche, 2004: Le réchauffement climatique récent: impact sur les arbres fruitiers et la vigne. Lett. PIGB-PMRC France Changement Global, 16, 50-54. [SRC]
Sekercioglu, C.H., G.C. Daily and P.R. Ehrlich, 2004: Ecosystem consequences of bird declines. P. Natl. Acad. Sci. USA, 101, 18042-18047. [JoC]
Selvaraju, R., 2003: Impact of El Niño-Southern Oscillation on Indian foodgrain production. Int. J. Climatol., 23, 187-206. [JoC]
Semenov, S.M., V.V. Yasukevich and E.S. Gel’ver, 2006: Identification of Climatogenic Changes. Publishing Center, Meteorology and Hydrology, Moscow, 325 pp. [NPR, ARC]
Serreze, M., D. Bromwich, M. Clark, A. Etringer, T. Zhang and R. Lammers, 2002: Large scale hydroclimatology of the terrestrial Arctic drainage system. J. Geophys. Res.–Atmos., 108, 8160. Clean
Shanks, G.D., S.I. Hay, D.I. Stern, K. Biomndo and R.W. Snow, 2002: Meteorologic influences on Plasmodium falciparum malaria in the highland tea estates of Kericho, western Kenya. Emerg. Infect. Dis., 8, 1404-1408. Clean
Shimaraev, M.N. and V.M. Domysheva, 2004: Climate and processes in Lake Baikal ecosystem at present. The VIth All-Russia Hydrological Congress: Abstracts of Papers 4, 287-288 (in Russian). [NPR]
Shimoda, Y., 2003: Adaptation measures for climate change and the urban heat island in Japan’s built environment. Build. Res. Inf., 31, 222-230. Clean
Simmons, A.D. and C.D. Thomas, 2004: Changes in dispersal during species’ range expansions. Am. Nat., 164, 378. Clean
Singh, B. and A.E. Fouladi, 2003: Coastal erosion in Trinidad in the southern Caribbean: probable causes and solutions. Coastal Engineering VI: Computer Modelling and Experimental Measurements of Seas and Coastal Regions, C.A. Brebbia, D. Almorza and F. López-Aguayo, Eds., WIT Press, Southampton, 397-406. [NPR, MoS]
Smith, L.C., 2000: Trends in Russian Arctic river-ice formation and breakup, 1917 to 1994. Phys. Geogr., 21, 46-56. Clean
Smith, L.C., Y. Cheng, G.M. MacDonald and L.D. Hinzman, 2005: Disappearing Arctic lakes. Science, 308, 1429. [JoC]
Smith, T.M. and R.W. Reynolds, 2005: A global merged land air and sea surface temperature reconstruction based on historical observations (1880-1997). J. Climate, 18, 2021-2036. [JoC]
Smith, V.R., 2002: Climate change in the sub-Antarctic: an illustration from Marion Island. Climatic Change, 52, 345-357. [JoC]
Smol, J.P., A.P. Wolfe, H.J.B. Birks, M.S.V. Douglas, V.J. Jones, A. Korhola, R. Pienitz, K. Ruhland, S. Sorvari, D. Antoniades, S.J. Brooks, M.A. Fallu, M. Hughes, B.E. Keatley, T.E. Laing, N. Michelutti, L. Nazarova, M. Nyman, A.M. Paterson, B. Perren, R. Quinlan, M. Rautio, E. Saulnier-Talbot, S. Siitoneni, N. Solovieva and J. Weckstrom, 2005: Climate-driven regime shifts in the biological communities of arctic lakes. P. Natl. Acad. Sci. USA, 102, 4397-4402. [PoC, JoC]
Sobrino, V.E., M.A. González, M. Sanz-Elorza, E. Dana, D. Sánchez-Mata and R. Gavilan, 2001: The expansion of thermophilic plants in the Iberian Peninsula as a sign of climate change. “Fingerprints” of Climate Change: Adapted Behaviour and Shifting Species Ranges, G.-R. Walther, C.A. Burga and P.J. Edwards, Eds., Kluwer Academic/Plenum, New York, 163-184. [NPR]
Sokolov, L.V., M.Y. Markovets, A.P. Shapoval and Y.G. Morozov, 1998: Long-term trends in the timing of spring migration of passerines on the Courish spit of the Baltic Sea. Avian Ecology and Behaviour, 1, 1-21. Clean
Sommaruga-Wograth, S., K.A. Koinig, R. Schmidt, R. Sommaruga, R. Tessadri and R. Psenner, 1997: Temperature effects on the acidity of remote alpine lakes. Nature, 387, 64-67. [JoC]
Sorvari, S., A. Korhola and R. Thompson, 2002: Lake diatom response to recent Arctic warming in Finnish Lapland. Glob. Change Biol., 8, 171-181. [JoC]
Southward, A.J., O. Langmead, N.J. Hardman-Mountford, J. Aiken, G.T. Boalch, P.R. Dando, M.J. Genner, I. Joint, M.A. Kendall, N.C. Halliday, R.P. Harris, R. Leaper, N. Mieszkowska, R.D. Pingree, A.J. Richardson, D.W. Sims, T. Smith, A.W. Walne and S.J. Hawkins, 2005: Long-term oceanographic and ecological research in the western English Channel. Adv. Mar. Biol., 47, 1-105. Clean
Spagnoli, B., S. Planton, M. Deque, O. Mestre and J.M. Moisselin, 2002: Detecting climate change at a regional scale: the case of France. Geophys. Res. Lett., 29, doi:10.1029/2001GL014619. [NPR, JoC]
Sparks, T.H. and R.G. Loxton, Eds.,1999: Arrival date of the swallow. Indicators of Climate Change in the UK. Centre for Ecology and Hydrology, Huntington, 62-63. [NPR, SRC]
Sparks, T.H. and O. Braslavska, 2001: The effect of temperature, altitude and latitude on the arrival and departure dates of the swallow Hirundo rustica in the Slovak Republic. Int. J. Biometeorol., 45, 212-216. [SRC]
Sparks, T.H. and A. Menzel, 2002: Observed changes in seasons: an overview. Int. J. Climatol., 22, 1715. [JoC, SRC]
Sparks, T.H., H. Heyen, O. Braslavska and E. Lehikoinen, 1999: Are European birds migrating earlier? BTO News, 223, 8. [SRC]
Sparks, T.H., E.P. Jeffree and C.E. Jeffree, 2000: An examination of the relationship between flowering times and temperature at the national scale using long-term phenological records from the UK. Int. J. Biometeorol., 44, 82-87. [SRC]
Steemers, K., 2003: Cities, energy and comfort: a PLEA 2000 review. Energ. Buildings, 35, 1-2. Clean
Stefanescu, C., J. Peñuelas and I. Filella, 2003: Effects of climatic change on the phenology of butterflies in the northwest Mediterranean Basin. Glob. Change Biol., 9, 1494-1506. [JoC]
Steininger, K.W. and H. Weck-Hannemann, Eds., 2002: Global Environmental Change in Alpine Regions: Recognition, Impact, Adaptation and Mitigation. Edward Elgar, Cheltenham, 296 pp. [NPR]
Stenseth, N.C., A. Mysterud, G. Ottersen, J.W. Hurrell, K.S. Chan and M. Lima, 2002: Ecological effects of climate fluctuations. Science, 297, 1292-1296. [JoC]
Stervander, M., K. Lindstrom, N. Jonzen and A. Andersson, 2005: Timing of spring migration in birds: long-term trends, North Atlantic Oscillation and the significance of different migration routes. J. Avian Biol., 36, 210-221. Clean
Stirling, I. and C.L. Parkinson, 2006: Possible effects of climate warming on selected populations of polar bears (Ursus maritimus) in the Canadian Arctic. Arctic, 59, 261-275. Clean
Stive, M.J.F., 2004: How important is global warming for coastal erosion? An editorial comment. Climatic Change, 64, 27-39. [JoC]
Stone, R.S., E.G., Dutton, J.M. Harris and D. Longenecker, 2002: Earlier spring snowmelt in northern Alaska as an indicator of climate change. J. Geophys. Res.–Atmos., 107, doi:10.1029/2000JD000286. [NPR]
Straile, D. and R. Adrian, 2000: The North Atlantic Oscillation and plankton dynamics in two European lakes: two variations on a general theme. Glob. Change Biol., 6, 663-670. [JoC]
Straile, D., K. Johns and H. Rossknecht, 2003: Complex effects of winter warming on the physicochemical characteristics of a deep lake. Limnol. Oceanogr., 48, 1432-1438. Clean
Strode, P.K., 2003: Implications of climate change for North American wood warblers (Parulidae). Glob. Change Biol., 9, 1137-1144. [JoC]
Sturm, M., C. Racine and K. Tape, 2001: Climate change: increasing shrub abundance in the Arctic. Nature, 411, 546-547. [JoC]
Su, Y.Z., H.L. Zhao, T.H. Zhang and X.Y. Zhao, 2004: Soil properties following cultivation and non-grazing of a semi-arid sandy grassland in northern China. Soil Till. Res., 75, 27-36. Clean
Sutherst, R.W., 2004: Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev., 17, 136. Clean
Svensson, C., J. Hannaford, Z.W. Kundzewicz and T.J. Marsh, 2006: Trends in river floods: why is there no clear signal in observations? IAHS/UNESCO Kovacs Colloquium: Frontiers in Flood Research, 305, 1-18. [NPR, ARC]
Swiss Reinsurance Company, 2005: Natural catastrophes and man-made disasters in 2004. Swiss Reinsurance Company. Economic Research and Consulting, Zürich, 40 pp. [NPR]
Sydeman, W.J., M.M. Hester, J.A. Thayer, F. Gress, P. Martin and J. Buffa, 2001: Climate change, reproductive performance and diet composition of marine birds in the southern California Current system, 1969-1997. Prog. Oceanogr., 49, 309-329. Clean
Syvitski, J.P.M., N. Harvey, E. Wolanski, W.C. Burnett, G.M.E. Perillo, V. Gornitz, 2005a: Dynamics of the coastal zone. Coastal Fluxes in the Anthropocene, C.J. Crossland, H.H. Kremer, H.J. Lindeboom, J.I.M. Crossland and M.D.A. Le Tissier, Eds., Springer, Berlin, 39-94. [NPR, SRC]
Syvitski, J.P.M., C.J. Vorosmarty, A.J. Kettner and P. Green, 2005b: Impact of humans on the flux of terrestrial sediment to the global coastal ocean. Science, 308, 376-380. [JoC]
Tamis, W.L.M., M. Van’t Zelfde and R. Van der Meijden, 2001: Changes in vascular plant biodiversity in the Netherlands in the 20th century explained by climatic and other environmental characteristics. Long-term Effects of Climate Change on Biodiversity and Ecosystem Processes, H. Van Oene, W.N. Ellis, M.M.P.D. Heijmans, D. Mauquoy, W.L.M. Tamis, F. Berendse, B. Van Geel, R. Van der Meijden and S.A. Ulenberg, Eds., NOP, Bilthoven, 23-51. [NPR]
Tank, A.M.G.K. and G.P. Konnen, 2003: Trends in indices of daily temperature and precipitation extremes in Europe, 1946-99. J. Climate, 16, 3665-1680. [JoC]
Taylor, J.A., A.P. Murdock and N.I. Pontee, 2004: A macroscale analysis of coastal steepening around the coast of England and Wales. Geogr. J., 170, 179-188. Clean
Teranishi, H., Y. Kenda, T. Katoh, M. Kasuya, E. Oura and H. Taira, 2000: Possible role of climate change in the pollen scatter of Japanese cedar Cryptomeria japonica in Japan. Climate Res., 14, 65-70. Clean
Thampanya, U., J.E. Vermaat, S. Sinsakul and N. Papapitukkul, 2006: Coastal erosion and mangrove progradation of southern Thailand. Estuar. Coast. Shelf Sci., 68, 75-85. Clean
Theurillat, J.P. and A. Guisan, 2001: Potential impact of climate change on vegetation in the European Alps: a review. Climatic Change, 50, 77-109. [JoC, ARC]
Thomas, C.D., 2005: Recent evolutionary effects of climate change. Climate Change and Biodiversity, T.E. Lovejoy and L. Hannah, Eds., Yale University Press, New Haven, Connecticut, 75-90. [NPR]
Thomas, C.D., E.J. Bodsworth, R.J. Wilson, A.D. Simmons, Z.G. Davies, M. Musche and L. Conradt, 2001a: Ecological and evolutionary processes at expanding range margins. Nature, 411, 577-581. [JoC]
Thomas, C.D., A. Cameron, R.E. Green, M. Bakkenes, L.J. Beaumont, Y.C. Collingham, B.F.N. Erasmus, M.F. de Siqueira, A. Grainger, L. Hannah, L. Hughes, B. Huntley, A.S. van Jaarsveld, G.F. Midgley, L. Miles, M.A. Ortega-Huerta, A.T. Peterson, O.L. Phillips and S.E. Williams, 2004: Extinction risk from climate change. Nature, 427, 145-148. [JoC, ARC]
Thomas, C.D., A.M.A. Franco and J.K. Hill, 2006: Range retractions and extinction in the face of climate warming. Trends Ecol. Evol., 21, 415-416. Clean
Thomas, D., H. Osbahr, C. Twyman, N. Adger and B. Hewitson, 2005: ADAPTIVE: Adaptations to climate change amongst natural resource-dependant societies in the developing world – across the Southern African climate gradient. Research Technical Report 35, Tyndall Centre for Climate Change, Norwich, 47 pp. [NPR, ARC]
Thomas, D.S.G. and C. Twyman, 2005: Equity and justice in climate change adaptation amongst natural resource-dependant societies. Global Environ. Chang., 15, 115-124. [JoC]
Thomas, D.W., J. Blondel, P. Perret, M.M. Lambrechts and J.R. Speakman, 2001b: Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science, 291, 2598-2600. [JoC]
Thompson, L.G., E. Mosley-Thompson, M.E. Davis, P.N. Lin, K. Henderson and T.A. Mashiotta, 2003: Tropical glacier and ice core evidence of climate change on annual to millennial time scales. Climatic Change, 59, 137-155. [JoC]
Tidemann, C.R., M.J. Vardon, R.A. Loughland and P.J. Brocklehurst, 1999: Dry season camps of flying-foxes (Pteropus spp.) in Kakadu World Heritage Area, north Australia. J. Zool., 247, 155-163. Clean
Tillman, D., J. Fargione, B. Wolff, C. D’Antonio, A. Dobson, R. Howarth, D. Schindler, W. Schlesinger, D. Simberloff and D. Swackhamer, 2001: Forecasting agriculturally driven global environmental change. Science, 292, 281-284. [JoC, MoS]
Trenberth, K.E., P.D. Jones, P.G. Ambenje, R. Bojariu, D.R. Easterling, A.M.G. Klein Tank, D.E. Parker, J.A. Renwick, F. Rahimzadeh, M.M. Rusticucci, B.J. Soden and P.-M. Zhai, 2007: Observations: surface and atmospheric climate change. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller, Eds., Cambridge University Press, Cambridge, 235-336. [NPR, PoC, 2007]
Trigo, R.M., R. Garcia-Herrera, J. Diaz, I.F. Trigo and M.A. Valente, 2005: How exceptional was the early August 2003 heatwave in France? Geophys. Res. Lett., 32, L10701, doi:10.1029/2005GL022410. [JoC]
Tryjanowski, P., 2002: A long-term comparison of laying date and clutch size in the red-backed shrike (Lanius collurio) in Silesia, southern Poland. Acta Zool. Acad. Sci. H., 48, 101-106. [SRC]
Tryjanowski, P., M. Rybacki and T. Sparks, 2003: Changes in the first spawning dates of common frogs and common toads in western Poland in 1978-2002. Ann. Zool. Fenn., 40, 459-464. [SRC]
Tryjanowski, P., T.H. Sparks, J. Ptaszyk and J. Kosicki, 2004: Do white storks Ciconia ciconia always profit from an early return to their breeding grounds? Bird Study, 51, 222-227. [SRC]
Tryjanowski, P., T.H. Sparks and P. Profus, 2005: Uphill shifts in the distribution of the white stork Ciconia ciconia in southern Poland: the importance of nest quality. Diversity and Distributions, 11, 219. [SRC]
Tulu, A.N., 1996: Determinants of malaria transmission in the highlands of Ethiopia: the impact of global warming on morbidity and mortality ascribed to malaria. PhD thesis, University of London, London, 301 pp. [NPR]
Turalioglu, F.S., A. Nuhoglu and H. Bayraktar, 2005: Impacts of some meteorological parameters on SO2 and TSP concentrations in Erzurum, Turkey. Chemosphere, 59, 1633-1642. Clean
Umina, P.A., A.R. Weeks, M.R. Kearney, S.W. McKechnie and A.A. Hoffmann, 2005: A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science, 308, 691-693. [JoC]
United Nations Development Programme, 2004: Reducing Disaster Risk: A Challenge for Development. United Nations Development Programme, New York, 161 pp, http://www.undp.org/bcpr/disred/rdr.htm. [NPR]
USGS, 2006: Land area changes in coastal Louisiana after the 2005 hurricanes. USGS Open-File Report 2006-1274, 2 pp. [NPR]
Valiela, I., J.L. Bowen and J.K. York, 2001: Mangrove forests: one of the world’s threatened major tropical environments. BioScience, 51, 807-815. Clean
van der Wal, D. and K. Pye, 2004: Patterns, rates and possible causes of saltmarsh erosion in the Greater Thames area (UK). Geomorphology, 61, 373-391. Clean
Van Duivenbooden, N., S. Abdoussalam and A.B. Mohamed, 2002: Impact of climate change on agricultural production in the Sahel. Part 2. Case study for groundnut and cowpea in Niger. Climatic Change, 54, 349-368. [JoC]
van Noordwijk, A.J., R.H. McCleery and C.M. Perrins, 1995: Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. J. Anim. Ecol., 64, 451-458. Clean
Van Vliet, A.J.H., A. Overeem, R.S. De Groot, A.F.G. Jacobs and F.T.M. Spieksma, 2002: The influence of temperature and climate change on the timing of pollen release in the Netherlands. Int. J. Climatol., 22, 1757-1767. [JoC]
Vare, H., R. Lampinen, C. Humphries and P. Williams, 2003: Taxonomic diversity of vascular plants in the European alpine areas. Alpine Biodiversity in Europe: A Europe-wide Assessment of Biological Richness and Change, L. Nagy, G. Grabherr, C. Körner and D.B.A. Thompson, Ecological Studies 167, Springer, Heidelberg, 133-148. [NPR]
Verburg, P., R.E. Hecky and H. Kling, 2003: Ecological consequences of a century of warming in Lake Tanganyika. Science, 301, 505-507. [JoC]
Verheye, H.M., A.J. Richardson, L. Hutchings, G. Marska and D. Gianakouras, 1998: Long-term trends in the abundance and community structure of coastal zooplankton in the southern Benguela system, 1951-1996. S. Afr. J. Mar. Sci., 19, 317-332. Clean
Vesely, J., V. Majer, J. Kopacek and S.A. Norton, 2003: Increasing temperature decreases aluminium concentrations in Central European lakes recovering from acidification. Limnol. Oceanogr., 48, 2346-2354. Clean
Vincent, W.F., J.A.E. Gibson and M.O. Jeffries, 2001: Ice-shelf collapse, climate change and habitat loss in the Canadian high Arctic. Polar Rec., 37, 133-142. Clean
Vollmer, M.K., H.A. Bootsma, R.E. Hecky, G. Patterson, J.D. Halfman, J.M. Edmond, D.H. Eccles and R.F. Weiss, 2005: Deep-water warming trend in Lake Malawi, East Africa. Limnol. Oceanogr., 50, 727-732. Clean
Vuille, M., R.S. Bradley, M. Werner and F. Keimig, 2003: 20th century climate change in the tropical Andes: observations and model results. Climatic Change, 59, 75-99. [JoC, MoS]
Wall, G., 1998: Implications of global climate change for tourism and recreation in wetland areas. Climatic Change, 40, 371-389. [JoC]
Walther, G.-R., 2000: Climatic forcing on the dispersal of exotic species. Phytocoenologia, 30, 409. [MoS]
Walther, G.-R., 2004: Plants in a warmer world. Perspect. Plant Ecol., 6, 169-185. Clean
Walther, G.-R., E. Post, P. Convey, A. Menzel, C. Parmesan, T.J.C. Beebee, J.M. Fromentin, O. Hoegh-Guldberg and F. Bairlein, 2002: Ecological responses to recent climate change. Nature, 416, 389-395. [JoC, SRC]
Walther, G.-R., S. Beissner and C.A. Burga, 2005a: Trends in the upward shift of alpine plants. J. Veg. Sci., 16, 541-548. Clean
Walther, G.-R., S. Berger and M.T. Sykes, 2005b: An ecological ‘footprint’ of climate change. P. Roy. Soc.Lond. B Bio., 272, 1427-1432. Clean
Warren, M.S., J.K. Hill, J.A. Thomas, J. Asher, R. Fox, B. Huntley, D.B. Roy, M.G. Telfer, S. Jeffcoate, P. Harding, G. Jeffcoate, S.G. Willis, J.N. Greatorex-Davies, D. Moss and C.D. Thomas, 2001: Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature, 414, 65-69. [JoC]
Watson, R.T. and W. Haeberli, 2004: Environmental threats, mitigation strategies and high mountain areas. Mountain Areas: A Global Resource. Ambio, 13, 2-10. [SRC]
Webster, P.J., G.J. Holland, J.A. Curry and H.R. Chang, 2005: Changes in tropical cyclone number, duration and intensity in a warming environment. Science, 309, 1844-1846. [JoC]
Weimerskirch, H., P. Inchausti, C. Guinet and C. Barbraud 2003: Trends in bird and seal populations as indicators of a system shift in the Southern Ocean. Antarct. Sci., 15, 249-256. Clean
Weissflog, L., N. Elansky, E. Putz, G. Krueger, C.A. Lange, L. Lisitzina and A. Pfennigsdorff, 2004: Trichloroacetic acid in the vegetation of polluted and remote areas of both hemispheres. Part II. Salt lakes as novel sources of natural chlorohydrocarbons. Atmos, Environ,, 38, 4197-4204. Clean
Weladji, R. and Ø. Holand, 2003: Global climate change and reindeer: effects of winter weather on the autumn weight and growth of calves. Oecologia, 136, 317-323. Clean
Werrity, A., A. Black, R. Duck, B. Finlinson, N. Thurston, S. Shackley and D. Crichton 2002: Climate Change: Flooding Occurrences Review. Scottish Executive Central Research Unit, Edinburgh, 94 pp. [NPR]
West, C.C. and M.J. Gawith, Eds., 2005: Measuring Progress: Preparing for Climate Change Through UK Climate Impacts Programme. UKCIP, Oxford, 72 pp. [NPR]
Westerling, A.L., H.G. Hidalgo, D.R. Cayan and T.W. Swetnam, 2006: Warming and earlier spring increases Western U.S. forest fire activity. Science, 313, 940-943. [JoC, ARC]
Weyhenmeyer, G.A., 2001: Warmer winters: are planktonic algal populations in Sweden’s largest lakes affected? Ambio, 30, 565-571. Clean
Weyhenmeyer, G.A., T. Blenckner and K. Pettersson, 1999: Changes of the plankton spring outburst related to the North Atlantic Oscillation. Limnol. Oceanogr., 44, 1788-1792. Clean
WHO, 2003: Phenology and human health: allergic disorders. Report on a WHO meeting, Rome. World Health Organization, Rome, 64 pp. [NPR]
Wilkinson, J.W., 2004. Status of Coral Reefs of the World. Australian Institute of Marine Science, Townsville, 580 pp. [NPR]
Williams, D.W. and A.M. Liebhold, 2002: Climate change and the outbreak range of two North American bark beetles. Agr. Forest Entomol., 4, 87-99. Clean
Williams, S.E., E.E. Bolitho and S. Fox, 2003: Climate change in Australian tropical rainforests: an impending environmental catastrophe. P. Roy. Soc. Lond. B Bio., 270, 1887-1892. Clean
Wilmking, M., G.P. Juday, V.A. Barber and H.S.J. Zald, 2004: Recent climate warming forces contrasting growth responses of white spruce at treeline in Alaska through temperature thresholds. Glob. Change Biol., 10, 1724-1736. [JoC]
Wilson, R.J., C.D. Thomas, R. Fox, D.B. Roy and W.E. Kunin, 2004: Spatial patterns in species distributions reveal biodiversity change. Nature, 432, 393-396. [JoC]
Wilson, R.J., D. Gutierrez, J. Gutierrez, D. Martinez, R. Agudo and V.J. Monserrat, 2005: Changes to the elevational limits and extent of species ranges associated with climate change. Ecol. Lett., 8, 1346-1346. Clean
Winder, M. and D.E. Schindler, 2004a: Climate change uncouples trophic interactions in an aquatic system. Ecology, 85, 3178-3178. Clean
Winder, M. and D.E. Schindler, 2004b: Climatic effects on the phenology of lake processes. Glob. Change Biol., 10, 1844-1856. [JoC]
Wolanski, E., S. Spagnol and E.B. Lim, 2002. Fine sediment dynamics in the mangrove-fringed muddy coastal zone. Muddy Coasts of the World: Processes, Deposits and Function, T. Healy, Y. Wang and J.A. Healy, Eds., Elsevier Science, Amsterdam, 279-292. [NPR]
Wolfe, A.P. and B.B. Perren, 2001: Chrysophyte microfossils record marked responses to recent environmental changes in high- and mid-arctic lakes. Can. J. Bot., 79, 747-752. Clean
Wolfe, D.W., M.D. Schwartz, A.N. Lakso, Y. Otsuki, R.M. Pool and N.J. Shaulis, 2005: Climate change and shifts in spring phenology of three horticultural woody perennials in northeastern USA. Int. J. Biometeorol., 49, 303-309. Clean
Wolters, M., J.P. Bakker, M.D. Bertness, R.L. Jefferies and I. Moller, 2005: Saltmarsh erosion and restoration in south-east England: squeezing the evidence requires realignment. J. Appl. Ecol., 42, 844-851. [ARC]
Wong, P.P., 2003: Where have all the beaches gone? Coastal erosion in the tropics. Singapore. J. Trop. Geo., 24, 111-132. Clean
Woodworth, P.L. and D.L. Blackman, 2004: Evidence for systematic changes in extreme high waters since the mid-1970s. J. Climate, 17, 1190-1197. [JoC]
Woolf, D.K., P.G. Challenor and P.D. Cotton, 2002: Variability and predictability of the North Atlantic wave climate. J. Geophys. Res.–Oceans, 107, 3145, doi:10.1029/2001JC001124. [MoS]
Worrall, F., T. Burt and R. Shedden, 2003: Long term records of riverine dissolved organic matter. Biogeochemistry, 64, 165-178. Clean
Yamada, T., 1998: Glacier lake and its outburst flood in the Nepal Himalaya. Japanese Society of Snow and Ice, Tokyo, 1, 96. Clean
Yang, D.Q., D.L. Kane, L.D. Hinzman, X.B. Zhang, T.J. Zhang and H.C. Ye, 2002: Siberian Lena River hydrologic regime and recent change. J. Geophys. Res.–Atmos., 107(D23), 4694, doi:10.1029/2002JD002542. Clean
Yang, S.L., J. Zhang, J. Zhu, J.P. Smith, S.B. Dai, A. Gao and P. Li, 2005: Impact of dams on Yangtze River sediment supply to the sea and delta intertidal wetland response. J. Geophys. Res.-Earth, 110, F03006, doi:10.1029/2004JF000271. Clean
Ye, H.C. and M. Ellison, 2003: Changes in transitional snowfall season length in northern Eurasia. Geophys. Res. Lett., 30, 1252, doi:10.1029/2003GL016873. [JoC]
Yom-Tov, Y., 2003: Body sizes of carnivores commensal with humans have increased over the past 50 years. Funct. Ecol., 17, 323-327. Clean
Yom-Tov, Y. and S. Yom-Tov, 2004: Climatic change and body size in two species of Japanese rodents. Biol. J. Linn. Soc., 82, 263-267. Clean
Yoshikawa, K. and L.D. Hinzman, 2003: Shrinking thermokarst ponds and groundwater dynamics in discontinuous permafrost near Council, Alaska. Permafrost Periglac., 14, 151-160. Clean
Zhang, K.Q., B.C. Douglas and S.P. Leatherman, 2000: Twentieth-century storm activity along the US east coast. J. Climate, 13, 1748-1761. [JoC]
Zhang, K.Q., B.C. Douglas and S.P. Leatherman, 2004: Global warming and coastal erosion. Climatic Change, 64, 41-58. [JoC]
Zhang, X.B., K.D. Harvey, W.D. Hogg and T.R. Yuzyk, 2001: Trends in Canadian streamflow. Water Resour. Res., 37, 987-998. Clean
Zhang, X.B., F.W. Zwiers and P.A. Stott, 2006: Multimodel multisignal climate change detection at regional scale. J. Climate, 19, 4294-4307. [JoC, MoS]
Zheng, J.Y., Q.S. Ge and Z.X. Hao, 2002: Impacts of climate warming on plants phenophases in China for the last 40 years. Chinese Sci. Bull., 47, 1826-1831. Clean
Zhou, G., N. Minakawa, A. Githeko and G. Yan, 2004: Association between climate variability and malaria epidemics in the East African highlands. P. Natl. Acad. Sci. USA, 24, 2375-2380. [JoC, SRC]
Zhou, L., C.J. Tucker, R.K. Kaufmann, D. Slayback, N.V. Shabanov and R.B. Myneni, 2001: Variations in northern vegetation activity inferred from satellite data of vegetation index during 1981 to 1999. J. Geophys. Res.–Atmos., 106, 20069-20083. Clean
Zhou, L., R.K. Kaufmann, Y. Tian, R.B. Myneni and C.J. Tucker, 2003: Relation between interannual variations in satellite measures of northern forest greenness and climate between 1982 and 1999. J. Geophys. Res., 108, 4004, doi:10.1029/2002JD002510. [JoC]
Zimov, S.A., E.A. Schuur and F.S. Chapin III, 2006: Permafrost and the global carbon budget. Science, 312, 1612-1613.[JoC]
| Tag | Explanation | Occurrences | Percentages |
|---|---|---|---|
| PoC |
Person of Concern Key individual involved in CRU emails as defined in this spreadsheet. |
10 | 1% |
| JoC |
Journal of Concern A Journal which has published articles by one or more PoCs (Person of Concern) |
198 | 30% |
| MoS |
Model or Simulation Reference appears to be a model or simulation, not observation or experiment. |
33 | 5% |
| NPR |
Non Peer Reviewed Reference has no Journal or no Volume or no Pages or it has Editors. |
114 | 17% |
| SRC |
Self Reference Concern Author of a chapter containing references to own work. |
93 | 14% |
| ARC |
Paper authored or co-authored by person who is also in list of Authors of another chapter. |
54 | 8% |
| 2007 |
Paper dated 2007, when IPCC policy stated cutoff was December 2005 |
13 | 2% |
| Clean |
The reference was probably peer reviewed. |
264 | 40% |
NOTE: In the Summary of Reviewer Comments,
Accepted includes only those responses that were unambiguously Accepted.
There are other categories of responses that we have not yet quantified
due to inconsistencies of usage.
| Reviewer | Total Comments | Accepted | IPCC Roles | Papers |
|---|---|---|---|---|
| Reid, PC | 193 | 55 | 0 | 10 |
| Government of USA | 98 | 5 | 0 | 0 |
| Gillett, Nathan | 95 | 12 | 0 | 0 |
| Zwiers, FW | 57 | 7 | 0 | 9 |
| Douguedroit, A | 57 | 2 | 0 | 1 |
| Government of Argentina | 49 | 2 | 0 | 0 |
| Tank, AK | 43 | 2 | 0 | 0 |
| Goklany, IM | 41 | 1 | 0 | 15 |
| Union, European | 37 | 6 | 0 | 0 |
| Government of Australia | 34 | 4 | 0 | 0 |
| Government of Netherlands | 32 | 1 | 0 | 0 |
| Government of Sweden | 31 | 0 | 0 | 0 |
| Hegerl, GC | 27 | 9 | 0 | 4 |
| Stone, Daithi | 26 | 1 | 0 | 0 |
| Beggs, PJ | 21 | 3 | 1 | 3 |
| Thomas, CD | 20 | 3 | 0 | 20 |
| Balzter, H | 20 | 4 | 0 | 1 |
| Government of Ireland | 20 | 2 | 0 | 0 |
| Chuine, Isabelle | 19 | 3 | 0 | 0 |
| Khandekar, M | 19 | 4 | 0 | 1 |
| Parkinson, Claire | 17 | 3 | 0 | 0 |
| Marsh, Terry | 15 | 0 | 0 | 0 |
| Chen, XY | 15 | 1 | 0 | 4 |
| Harguindeguy, NP | 14 | 3 | 0 | 0 |
| Wilson, RJ | 13 | 1 | 0 | 6 |
| Stott, PA | 13 | 2 | 0 | 11 |
| Government of Pakistan | 12 | 1 | 0 | 0 |
| Palutikof, JP | 12 | 1 | 2 | 7 |
| Ribera, Pedro | 11 | 5 | 0 | 0 |
| Kaser, G | 10 | 1 | 1 | 6 |
| Milly, C | 9 | 0 | 1 | 0 |
| Reisinger, A | 9 | 2 | 0 | 1 |
| Nicholls, N | 9 | 0 | 0 | 18 |
| Anadon, R | 9 | 0 | 0 | 2 |
| Maracchi, G | 8 | 0 | 0 | 1 |
| Nadelhoffer, Knute | 8 | 1 | 0 | 0 |
| Groisman, PY | 6 | 1 | 0 | 3 |
| Government of Switzerland | 6 | 1 | 0 | 0 |
| Marshall, P | 6 | 0 | 0 | 4 |
| Government of Spain | 5 | 0 | 0 | 0 |
| Smith, SJ | 5 | 1 | 0 | 16 |
| Bernstein, Lenny | 5 | 0 | 0 | 0 |
| Bernier, Pierre | 5 | 0 | 0 | 0 |
| Hansen, LJ | 5 | 0 | 0 | 4 |
| Hoeppe, Peter | 4 | 1 | 0 | 0 |
| Gant, Mary | 4 | 2 | 0 | 0 |
| Reiter, P | 4 | 0 | 0 | 4 |
| Government of Norway | 4 | 1 | 0 | 0 |
| Lapin, Milan | 4 | 0 | 0 | 0 |
| Spencer, T | 4 | 0 | 1 | 4 |
| Penuelas, J | 3 | 0 | 0 | 25 |
| Government of Germany | 3 | 0 | 0 | 0 |
| Shafer, SL | 3 | 0 | 0 | 3 |
| Pauli, H | 3 | 2 | 0 | 8 |
| Republic of Korea | 3 | 0 | 0 | 0 |
| Gonzalez, P | 2 | 1 | 0 | 1 |
| Walther, Gian-Reto | 2 | 2 | 0 | 0 |
| Government of France | 2 | 0 | 0 | 0 |
| Government of Finland | 2 | 0 | 0 | 0 |
| Government of Belgium | 2 | 0 | 0 | 0 |
| Hedger, M | 1 | 0 | 0 | 2 |
| Petriccione, B | 1 | 0 | 0 | 1 |
| Kheshgi, HS | 1 | 0 | 0 | 3 |
| Hare, W | 1 | 0 | 0 | 2 |
| Forbes, DL | 1 | 0 | 5 | 14 |
| Levermore, GJ | 1 | 0 | 0 | 2 |
| van Aalst, MK | 1 | 0 | 0 | 10 |
| Government of Austria | 1 | 0 | 0 | 0 |
| Government of Canada | 1 | 0 | 0 | 0 |
| Jeffrey, Paul | 1 | 1 | 0 | 0 |
| Ceron, JP | 1 | 0 | 1 | 3 |
| Totals | 1226 | 160 | 12 | 229 |
| Reviewer Type | Total Comments | Accepted | % Accepted |
|---|---|---|---|
| Govt | 302 | 17 | 5% |
| IPCC | 611 | 101 | 16% |
| Indep | 313 | 42 | 13% |
| Total | 1226 | 160 | 13% |
Main AR4 Index | Working Group WG2 Index | Table of Contents | Authors | Executive Summary | Annotated Text | References | Reviewer Comments